August 29, 2011 — A German team has developed a completely new non-invasive method to identify heart failure. It consists of an "electronic nose" which could make the "smelling" of heart failure possible. The project was presented at the European Society of Cardiology (ESC) congress 2011. “The early detection of chronic heart failure (CHF) through periodical screening facilitates early treatment application,” said investigator Vasileios Kechagias from the University Hospital Jena.
Heart failure is a common, costly, disabling and potentially deadly condition. In developed countries, around 2 percent of adults suffer from it, but in those age 65 or older, this increases to 6–10 percent. It is also associated with high health expenditure, mostly due to costs of hospitalization. Heart failure is associated with significantly reduced physical and mental health, resulting in a markedly decreased quality of life. Although some people survive many years, progressive disease is associated with an overall increased mortality and morbidity.
“We conducted a daily screening of patients with different degrees of heart failure,” explained Kechagias. “For the study, eligible patients were enrolled after informed consent, and the collected data was anonymous. Measurements were made in collaboration with the University of Applied Sciences, Jena. The participating physicians of the Department of Internal Medicine I, University Hospital Jena, were responsible for patient recruitment and analysis of clinical data.”
In particular, the relevant laboratory parameters for heart failure (BNP, minerals, creatinine, blood gas analysis) were collected and a clinical assessment of heart failure based on the available parameters (clinical history, laboratory, echocardiography and exercise stress test) was performed. In 2010, a total of 250 patients were screened, including 126 in the clinical study. In the course of the study, testing was optimized through a standardized skin preparation. The assignment of patients to the different groups (no heart failure vs. moderate heart failure vs. decompensated heart failure) was performed by physicians blinded for the measured values through the electronic nose.
Two groups were formed with congestive heart failure (CHF) patients: one with decompensated (n=27) heart failure and one with compensated (n=25) heart failure. As clinical manifestation of the decompensated heart failure, investigators evaluated the marked limitation of any activity where the patient is comfortable only at rest (Class III) or the state in which any physical activity brings on discomfort and symptoms occur at rest (Class IV). Furthermore, they screened a control group of patients without heart failure symptoms (n=28). Then the measurement with the "electronic nose" randomly took place, from 10 cycles of 3 minutes each and a subsequent offline-data-analysis.
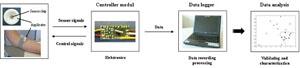
The “electronic nose” system consists of an array of three thick-film metal oxide-based gas sensors with heater elements. Each of the sensors has a slightly different sensitivity to various odorant molecular types. Interactions between molecules and the sensor are caused by reactions with oxygen on the heated sensor surface leading to a change of the free charge carrier concentrations and thus to a change in conductivity in the metal oxide layer. The odor components are divided by a statistical analysis into two principal components.
In all patients, data acquisition was possible. The patients with decompensated heart failure could be divided from compensated heart failure with 89 percent sensitivity and 88 percent specificity. Cardiovascular drug use was the same in these groups. On the other hand, patients without heart failure (control group) were different from the patients with heart failure in the principal-component analysis (89 percent sensitivity and 84 percent specificity).
Further work is in progress to identify the responsible components.
The primary objective is to create and establish a minimal invasive method, which will help to rapidly screen, diagnose, group and monitor the CHF.
For more information: www.escardio.org


 January 05, 2026
January 05, 2026 









