August 29, 2011— The echocardiographic response (reduction of left ventricular end-systolic volume), evaluated at six months follow-up, proved a better predictor of long-term mortality than clinical status improvement in a large population of cardiac resychronization therapy (CRT) patients. Therefore, assessment of occurrence of left ventricular reverse remodeling at mid-term follow-up may be an adequate surrogate endpoint in heart failure patients treated with CRT.
CRT efficacy has been demonstrated with significant reductions in mortality and morbidity of heart failure patients. Many studies have evaluated its efficacy via heart failure symptom improvement (clinical response) or left ventricular volume reduction (left ventricular reverse remodeling) at mid-term follow-up (3 or 6 months after CRT implantation). Echocardiographic response is also used for evaluation.
Based on these surrogate end points, the efficacy of CRT may change significantly and, consequentially, definition of response to CRT is still debated. Ideally, these surrogate end points should determine a significant reduction in mortality. Accordingly, the present evaluation investigated which definition of CRT response at mid-term follow-up best predicts long-term mortality.
A total of 663 advanced heart failure patients were followed-up for the occurrence of all-cause mortality. At six months, the clinical and echocardiographic responses to CRT were evaluated. Clinical response was defined as a reduction in New York Heart Association (NYHA) functional class of at least one point; echocardiographic response was defined by a reduction in left ventricular end-systolic volume of at least 15 percent. Based on these definitions, 510 (77 percent) patients showed clinical response and 412 (62 percent) patients showed echocardiographic response to CRT.
During a mean follow-up of 37±22 months, 140 (21 percent) patients died. Clinical and echocardiographic CRT responses were both significantly related to all-cause mortality. However, only echocardiographic response to CRT was independently associated with a superior survival. In particular, a patient not showing echocardiographic response had a risk of death three times higher than a patient showing a good echocardiographic response (hazard ratio 0.38; 95 percent confidence intervals, 0.27-0.50; p<0.001).
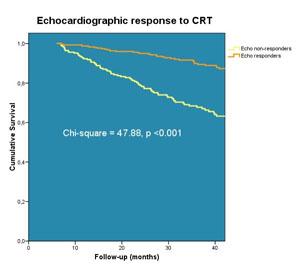
The figure shows the probability of all-cause mortality, which differed significantly between the two groups dichotomized based on the echocardiographic response (left ventricular reverse remodeling). A cumulative 1 percent, 4 percent and 8 percent of patients with LV reverse remodeling died by 12, 24 and 36 months follow-up, respectively. In contrast, a respective 8 percent, 19 percent and 27 percent of the patients without LV reverse remodeling died during the same time period (log-rank p <0.001).
The present findings have important implications in the design process of clinical trials. Use of biological markers in the prevention and progression of heart failure (such as changes in left ventricular volumes and function) allow investigators to make prompt evaluations of heart failure therapies; it also helps to understand the biologic processes underlying the disease and the mechanisms of the therapy. In addition, the use of these surrogate end points permits smaller sample size, shorter trial duration and reduced costs.
In conclusion, the echocardiographic response, evaluated at 6 months follow-up, demonstrated to be a better predictor of long-term mortality than improvement in clinical status in a large population of CRT patients. Therefore, assessment of occurrence of left ventricular reverse remodeling at mid-term follow-up may be an adequate surrogate end point in heart failure patients treated with CRT.
For more information: www.escardio.org


 January 05, 2026
January 05, 2026 









