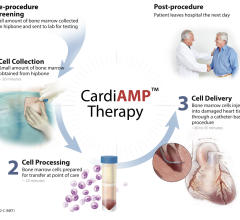
July 14, 2010 – A Belgian biotechnology company recently announced positive safety data and preliminary efficacy results from a clinical trial of its new stem cell therapy for heart failure.
Results of the Cardio3 BioSciences trial showed C-Cure to have a very good safety profile with no adverse events, as assessed by an independent board. The study is also examining a number of measures of efficacy. With three month follow up data in-hand, Cardio3 BioSciences has observed positive and encouraging trends in a number of physiological and clinical parameters. Meaningful differences were seen in ventricular size, ejection fraction and other measures of heart muscle activity in C-Cure treated patients when compared to control and baseline. Partial data from a paired analysis of patients at six-months follow-up is suggestive of these beneficial trends being reinforced over time. Cardio3 Biosciences intends to publish the study results once the full six-month dataset is available and has been analyzed.
“C-Cure could represent a major breakthrough in the field of cardiac regenerative medicine offering the potential of a life-saving treatment potentially avoiding the need for heart transplants,” said Jozef Bartunek, associate director of the Cardiovascular Center in Aalst, Belgium, and co-principal investigator of the C-Cure trial. “This trial represents a ‘first-in-man’ therapy using cells ‘programmed’ to become heart cells. The early stage data that we have seen are encouraging and provides us with very valuable insights that we can use in the design of larger studies to fully examine the efficacy of C-Cure in heart failure patients.”
The current C-Cure study is a randomized, prospective, multi-center trial to evaluate the safety and efficacy of C-Cure beyond optimal clinical care in patients with heart failure. It recruited 45 patients in Belgium and Serbia. The primary end point of the trial is change in left ventricular ejection fraction (a measure of how well the heart is functioning) at six months post treatment.
Using the insights from the trial and input from regulators in Europe and the US, Cardio3 BioSciences is now designing a pivotal clinical trial program for C-Cure expected to start in 2011. With the Phase II stage completed and to allow for potential modifications to the trial protocol, Cardio3 BioSciences will not continue recruitment into the existing trial but will continue to gather all data for the six month analysis.

 January 14, 2025
January 14, 2025