May 10, 2010 – A catheter-based procedure is providing relief to patients who suffer from shortness of breath and fluid overload as the result of a severely leaky heart valve, but who are too sick for open-chest surgery. This was the finding of a study presented last week at the Society for Cardiovascular Angiography and Interventions (SCAI) 33rd Annual Scientific Sessions.
Data from the Endovascular Valve Edge-to-Edge REpair STudy (EVEREST II) High Risk Registry showed that, in patients who are not healthy enough to undergo surgery to repair a leaky mitral valve, use of the MitraClip valve repair system is safe. In the majority of patients, it was found effective in relieving symptoms that limit the ability to engage in day-to-day activities. There has never been a treatment option for this high-risk group of patients in the past.
"The MitraClip system is a first-in-class treatment and a remarkable innovation – the beginning of a very exciting era for treating valvular disease with minimally invasive devices," said Saibal Kar, M.D., FSCAI, director of interventional cardiac research at the Heart Institute, Cedars Sinai Medical Center, Los Angeles.
Mitral Regurgitation
The mitral valve controls blood flow between the atrium and the ventricle on the left side of the heart. A leaky mitral valve, or mitral regurgitation, can develop when the valve flaps, or leaflets, do not close effectively. This can happen either because the valve flaps are scarred or defective, or because the heart has become enlarged, stretching the valve opening so much that the flaps no longer meet in the center.
Mitral regurgitation is the most common condition involving the valves of the heart. Of the 250,000 patients newly diagnosed with mitral regurgitation in the United States each year, only about 20 percent have surgery. The rest are either too high-risk or not yet sick enough for surgery.
Transcatheter Mitral Repair
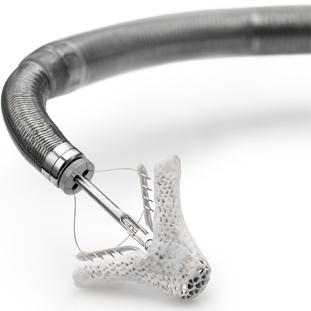
The MitraClip is an investigational device that has not yet been approved by the U.S. Food and Drug Administration (FDA). During the procedure, the device is mounted on a catheter and advanced into the heart via a vein in the groin. The clip is centered directly across the center of the valve and the leaflets are clipped together in the middle, converting a leaky single orifice mitral valve into a competent double-orifice valve. The entire procedure is done on a beating heart using X-ray and ultrasound to guide the procedure.
EVEREST II Study
For the study, interventional cardiologists recruited 78 patients with severe mitral regurgitation (classified as moderate-to-severe, or severe) who were considered to be at very high risk for surgery. The average age of patients was 77 years. All patients had congestive heart failure, and 90 percent were in New York Heart Association (NYHA) Class III or IV, meaning they were experiencing marked limitation of physical activity or even shortness of breath while resting. Some 60 percent of patients had previously undergone cardiac surgery, 85 percent had coronary artery disease, 35 percent had chronic lung disease, and 23 percent had moderate-to-severe kidney failure. According to a formula developed by the Society of Thoracic Surgeons, the average predicted risk of surgery-related death was 18.2 percent.
The placement of the MitraClip was successful in 96 percent of patients, with no procedural deaths. Within the first 30-days, mortality was 7.7 percent, which was significantly lower than the predicted 18.2 percent surgical mortality and not significantly different from the 8.3 percent mortality in a matched control group who were treated with medications alone. At 30-day follow-up, 75 percent of survivors were in NYHA Class I or II, meaning no or only slight limitation in physical activity. Survival at one year was 75 percent, better than the 55 percent in the medication-only control group.
At one year, 74 percent of survivors who were treated with the MitraClip were still in NYHA Class I or II and average heart function was improved by several measures. In addition, there was a 45 percent reduction in readmissions to the hospital for congestive heart failure. None of the patients in the study developed more severe symptoms, worse mitral regurgitation or underwent surgery within that time period.
Researchers concluded that treatment with the MitraClip valve repair system can be performed safely in high-risk patients and provides long-lasting relief of symptoms.
"Patients who couldn't walk across a room without being short of breath are now able to walk up a flight of stairs, go shopping, and exercise," said Kar. "The MitraClip procedure can be transformational for patients."
The study was sponsored by Evalve Inc, which is now part of Abbott. Kar received research grants from Evalve Inc.
For more information: www.scai.org


 January 05, 2026
January 05, 2026 









