Image courtesy of Sunshine Heart
January 21, 2015 — Sunshine Heart Inc. announced the company's German Erlangen site for the OPTIONS-HF study has implanted its first patient with the C-Pulse(R) System for moderate to severe heart failure. Michael Weyand, M.D., professor at the University Hospital of Erlangen performed the operation, which took place on November 19, 2014. The surgical implantation procedure was successfully completed and the patient suffering from an ischemic cardiomyopathy was discharged from the hospital eight days post-surgery.
"The study results showed improvement of heart failure symptoms without the patient requiring anticoagulation medication for the device. Furthermore, we preserve the option for a VAD implantation or heart transplant,” commented Daniel Bujnoch, resident of the Cardiac Surgery Department of the University Hospital of Erlangen.
This marks the 12th implantation of the CE marked C-Pulse System across fourteen activated EU centers participating in the OPTIONS HF post-market surveillance clinical study.
The OPTIONS HF study is a post-market, multi-center, prospective, open label study that will include 50 patients in up to 15 European centers. The study is designed to observe clinical outcomes of heart failure patients treated with the C-Pulse system. The primary endpoint is comparable to the COUNTER HF study as it evaluates the rate of re-hospitalization due to worsening heart failure and heart failure related death in addition to many other traditional heart failure endpoints.
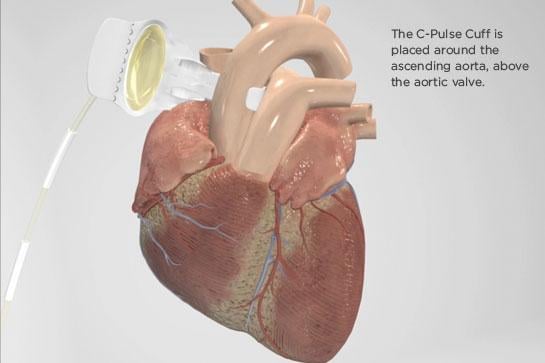
The C-Pulse Heart Assist System, or C-Pulse System, an investigational device in the United States, Canada and countries that do not recognize the CE mark approval, utilizes the scientific principles of intra-aortic balloon counterpulsation applied in an extra-aortic approach to assist the left ventricle by reducing the workload required to pump blood throughout the body, while increasing blood flow to the coronary arteries. Combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process, thereby potentially preventing the need for later-stage heart failure devices, such as left ventricular assist devices (LVADs), artificial hearts or transplants. It may also provide relief from the symptoms of Class III and ambulatory Class IV heart failure and improve quality of life and cardiac function. Based on the results from the feasibility study, some patients treated with the C-Pulse System will be able to stop using the device due to sustained improvement in their conditions as a result of the therapy.
For more information: www.sunshineheart.com


 January 05, 2026
January 05, 2026 









