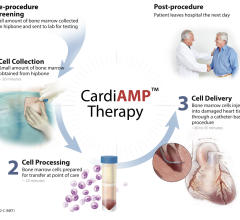
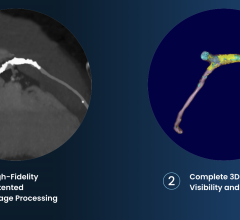
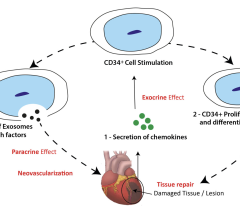
January 26, 2011 - The University of Michigan Cardiovascular Center and the University of Pittsburgh have been awarded $13.3 million to explore the potential benefits of heart devices for the large and growing group of Americans with heart failure.
The National Heart, Lung and Blood Institute (NHLBI) and HeartWare, a maker of left ventricular assist devices (LVAD), are sponsoring the study of earlier access to these devices that support the circulation of patients with failing hearts. In REVIVE-IT, researchers will compare whether non-transplant eligible patients with heart failure less advanced than that of current LVAD recipients do better with implanted devices than with current medical therapy.
"The new study allows us to examine the use of heart devices earlier in the cascade of heart failure," said Keith Aaronson, M.D., M.S., medical director of the heart transplant program and Center for Circulatory Support at the U-M Cardiovascular Center. Aaronson is also a principle investigator of the study.
For most patients, either a past heart attack or certain conditions such as hypertension, heart muscle diseases, abnormal heart valves or diabetes has lead to heart failure.
LVADs are currently used in patients with very advanced heart failure as a last resort to help them survive the wait for a heart transplant, or serve as a permanent alternative to heart transplantation.
"In REVIVE-IT we'll test the theory that heart failure patients whose condition impairs their daily lives, but who have not suffered serious consequences such as organ damage, malnourishment or immobility, would benefit from earlier implantation of an LVAD," said Robert Kormos, M.D., director of the UPMC artificial heart program and co-director of the UPMC heart transplantation program. Kormos is also one of the study’s principle investigators.
"Ventricular assist devices have been shown to improve both the quality and length of life of late-stage heart failure patients," said J. Timothy Baldwin, Ph.D., REVIVE-IT trial project officer, division of cardiovascular sciences, NHLBI. "This trial promises to help us learn if there are advantages to providing these devices before patients reach late-stage heart failure."
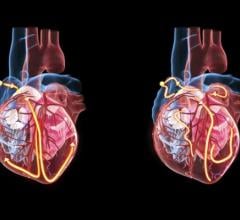
The REVIVE-IT study will use HeartWare's HVAD pump left ventricular assist device, a battery-operated continuous blood flow pump that's surgically placed within the heart and the pericardial space.
The pilot study will include 100 patients from selected sites across the United States, including the U-M and Pittsburgh. Site selection for the study will begin later this year. The U-M's Michigan Institute for Clinical and Health Research will coordinate the study.
"Our work may advance the treatment of heart failure by evaluating whether technology now reserved for very severe heart failure is ready for application to a broader group of patients in need," said Francis A. Pagani, M.D., Ph.D., surgical director of the heart transplant program and the Center for Circulatory Support at the U-M. Pagani is also a principal investigator of the study.
For more information: www.heartware.com, www.nhlbi.nih.gov

 January 14, 2025
January 14, 2025