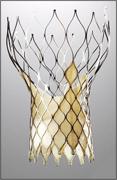
February 18, 2011 – An independent study in the journal Circulation confirmed the early and sustained clinical benefits for a system treating aortic valve disease. The article showed that patients receiving the CoreValve system, from Medtronic, have high rates of procedural success (98 percent), low rates of adverse events (2.1 percent) and substantial improvements of cardiac function.
The study, which was the largest CoreValve clinical study to date, looked at 663 consecutive aortic stenosis patients (mean age of 81 years) treated at 14 Italian centers beginning immediately after device commercialization in 2007.
The system is a minimally invasive, non?surgical treatment option for patients requiring aortic valve replacement who are considered at high risk for surgery. It is delivered via transcatheter aortic valve implantation (TAVI) rather than open?heart surgery. It received CE (Conformité Européenne) mark in March 2007 and has been implanted in more than 12,000 patients worldwide in 42 countries outside the United States. In the United States, the system is being evaluated in a U.S. pivotal clinical trial.
“The positive results of our TAVI experience are encouraging and demonstrate positive clinical outcomes for a group of patients with few treatment options,” said Corrado Tamburino, M.D., Ph.D., of Ferrarotto Hospital in Catania, Italy and principal investigator of the study. “Our findings demonstrate that a large majority of patients were treated successfully with CoreValve and experience improvements in heart function. This real?life registry gives further credence to this new therapy approach.”
In this study, procedural success was 98 percent and intraprocedural mortality was 0.9 percent. Cumulative incidences of mortality were 5.4 percent at 30 days, 12.2 percent at six months, and 15 percent at one year. Additionally, the study reported low rates of permanent pacemaker implantation (16.6 percent at two weeks and 17.4 percent at 30 days). There also was substantial and sustained improvement in patients’ health status, with 71.5 percent of patients in NYHA Class III and IV before the implant achieving Class II after the procedure.
The Italian Registry data also evaluated the incidence and predictors of early (30 days) and late (30 days to one year) mortality after TAVI. The authors concluded that while early mortality was strongly associated with procedural complications, late mortality was associated with comorbidities (such as a prior stroke, a prior episode of acute pulmonary edema, or chronic kidney disease) and that pacemaker implant was not a factor.
For more information: www.medtronic.com


 January 05, 2026
January 05, 2026 









