January 4, 2010 – The first patient was enrolled the TAXUS Liberte arm of the Dual Anti-Platelet Therapy (DAPT) Study, evaluating the TAXUS Liberté stent in combination with a dual anti-platelet therapy drug that includes aspirin and Effient.
Co-sponsors of the study include Eli Lilly and Company and Daiichi Sankyo Inc., manufacturers of Effient, which was recently approved by the FDA. The first patient was enrolled by Joel Cohn, M.D., FACC, at the Ingham Regional Medical Center in Lansing, Mich.
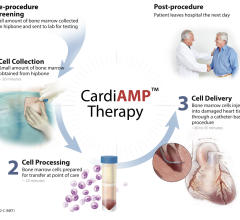
This is the first TAXUS Liberte post-approval study. It will enroll about 4,200 patients at up to 65 U.S. sites. The study will evaluate clinical outcomes in a broad range of patients with coronary artery disease who receive a TAXUS Liberte drug eluting stent followed by the use of aspirin and Effient (prasugrel). The primary endpoint is the rate of cardiac death or myocardial infarction (MI) at 12 months. Secondary endpoints will be analyzed out to five years and include rates of stent thrombosis using the Academic Research Consortium (ARC) definition, target vessel failure (TVF), target vessel revascularization (TVR), MI, bleeding events and stroke.
Boston Scientific plans to contribute data on the first 1,524 eligible patients from the TAXUS Liberte study to the DAPT Study, a collaboration among the FDA, drug and device manufacturers, and the Harvard Clinical Research Institute (HCRI). This four-year public health study will investigate the appropriate duration of dual anti-platelet therapy following drug-eluting stent implantation. HCRI is responsible for the scientific management and independent analysis of the overall study.
For more information: www.bostonscientific.com, www.hcri.harvard.edu

 January 14, 2025
January 14, 2025