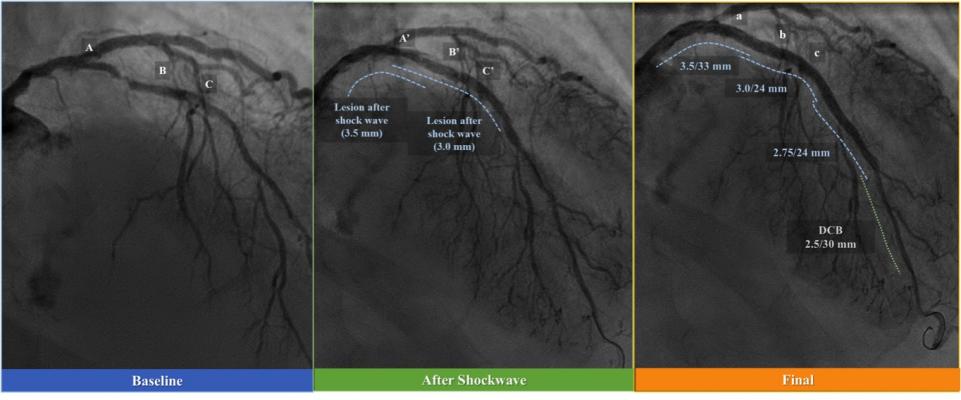
January 16, 2019 — Shockwave Medical Inc. has initiated its U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) study – DISRUPT CAD III – for the use of intravascular lithotripsy (IVL) in heavily calcified coronary arteries. IVL is a lesion preparation tool designed to fracture problematic calcium using sonic pressure waves in order to facilitate stent delivery, deployment and expansion.
The co-principal investigators of the study are:
- Dean Kereiakes, M.D., FACC, FSCAI, medical director of The Christ Hospital Heart and Vascular Center and The Lindner Research Center in Cincinnati, Ohio and professor of clinical medicine, The Ohio State University; and
- Jonathan Hill, M.D., consultant cardiologist at King’s College Hospital in London.
The first patient was enrolled last week by Richard A. Shlofmitz, M.D., FACC, chairman, Department of Cardiology, St. Francis Hospital in Roslyn, N.Y.
Coronary artery calcium physically impairs stent expansion, and is perhaps the single most important predictor of early stent thrombosis and restenosis after stent procedures. Current calcium modification treatments, which can be difficult to perform, only address the burden of intimal calcium with varying degrees of success and result in an increased risk for adverse events since these techniques do not differentiate between the calcific lesion and soft, normal intimal tissue.
Coronary IVL is a novel investigational therapy designed to treat calcified artery blockages with sonic pressure waves historically used to treat patients with kidney stones. The technology seeks to minimize trauma within the artery by delivering pulsatile sonic pressure waves locally to fracture both intimal and medial calcium in the artery wall but pass through surrounding soft vascular tissue in a safe manner.
“After previously using the peripheral IVL technology – Shockwave M5 – to enable transfemoral access for TAVR [transcatheter aortic valve replacement] as well as for mechanical cardiac support and hearing the enthusiasm from Europe about coronary IVL, we are very excited to investigate the clinical potential of the coronary technology in the United States,” said Kereiakes. “This therapy holds tremendous potential from a safety perspective for patients and an ease of use perspective for physicians – if coronary IVL is shown to be safe and effective, it could be a game changer for the way we treat calcified arteries today.”
DISRUPT CAD III is a prospective, non-randomized, multicenter global IDE study to demonstrate the safety and effectiveness of the Shockwave Coronary IVL System with the Shockwave C2 Coronary IVL Catheter in de novo, calcified, stenotic, coronary arteries prior to stenting. The study is expected to enroll approximately 392 patients at 50 global centers in the United States and Europe.
The study will assess the freedom from major adverse cardiac events (MACE) within 30 days of the index procedure as the primary safety endpoint. The primary effectiveness endpoint is procedural success which, based on predicate studies, is defined as stent delivery with a residual stenosis of less than 50 percent and without in-hospital MACE. Enrolled study patients will be followed for two years.
The study’s chairman is Gregg W. Stone, M.D., FACC, FSCAI, professor of medicine at Columbia University Medical Center in New York, and the angiographic and optical coherence tomography (OCT) core labs are the Cardiovascular Research Foundation, also based in New York.
“Having treated nearly 100 patients with IVL since its European launch, the benefits for treating complex patients are evident. I am delighted to be a part of this important global trial to introduce my U.S. interventional colleagues to this novel technology. IVL is easy to use and has been a huge advancement for our management of calcified lesions,” said Hill. “I think U.S. interventionalists will recognize the simplicity and ease of use of the IVL system and will appreciate its ability to be rapidly deployed in any cath lab. I have no doubt that IVL is poised to become the key differentiating technology compared to other calcium modification tools.”
Shockwave C2 Coronary IVL catheters are commercially available for the treatment of de novo coronary artery disease in Europe and select other geographies; they are limited to investigational use in the United States. Shockwave M5 Peripheral IVL catheters are commercially available for treatment of peripheral artery disease, including iliac arteries, in the United States, Europe and select other geographies.
For more information: www.shockwavemedical.com


 January 05, 2026
January 05, 2026 









