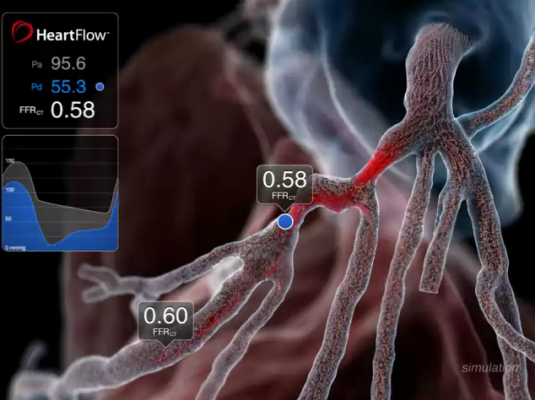
September 11, 2012 — Data presented at the 2012 European Society of Cardiology (ESC) Congress in Munich, Germany, from the prospective Determination of Fractional Flow Reserve by Anatomic Computed Tomographic Angiography (DeFACTO) study show that, when compared to standard coronary angiography, the noninvasive assessment of fractional flow reserve by computed tomography (FFR-CT) provides a more accurate determination of which lesions require invasive evaluation.[1] The results of the study were presented by James K. Min, director of cardiac imaging research and co-director of cardiac imaging at department of medicine, imaging and biomedical sciences at Cedars-Sinai Heart Institute in Los Angeles.
The study compared the ability of FFR-CT and computed tomography (CT) alone to identify flow-restricting lesions by assessment of fractional flow reserve (FFR), a measurement recognized as the standard for determining which lesions require treatment.[2] DeFACTO enrolled 252 stable patients with suspected coronary artery disease at 17 centers in five countries. All patients underwent CT, invasive coronary angiography, invasive FFR and subsequent FFR-CT analysis.
Results showed that FFR-CT was better able to identify flow-restricting arterial lesions than CT alone. The per-patient sensitivity and specificity of FFR-CT were also higher than CT alone using an area under the curve (AUC) analysis (AUC 0.81 vs. 0.68, p=0.0002).
The improvement in diagnostic performance was found greatest in arterial blockages of intermediate severity. In this set of patients, there was over a two-fold increase in test sensitivity, from 37 to 82 percent, with no loss of specificity. In these patients, the AUC improved from 0.53 for CT alone to 0.80 for FFR-CT (p=0.0002).
"One of the central challenges in taking care of patients with coronary artery disease is knowing which ones need further invasive evaluation for determining the need for coronary revascularization," said Min. "The results of the DeFACTO trial clearly demonstrate that when added to coronary CT angiographic findings, FFR-CT provides essential physiologic information as to which specific arterial blockages truly restrict blood flow to the heart and heighten patient risk.
"This is an exciting step forward for cardiology that could significantly improve how we guide patients towards the most effective and efficient care. Our findings also suggest that FFR-CT could be particularly useful for evaluating patients with arterial blockages of an intermediate severity, which are often the most difficult to assess noninvasively. This represents a large group of patients who unfortunately are often prone to frequent misdiagnosis. In addition, given the high negative predictive value of FFR-CT, it may serve as an effective 'gatekeeper' to further unnecessary invasive procedures."
For more information: www.escardio.org
References
1. The HeartFlow technology investigated in the study is a Web-based service that enables the computation of noninvasive FFR and thereby the identification of which lesions are causing ischemia. The technology computes FFR-CT from patient-specific 3-D computational models of the aorta, heart and coronary artery tree obtained from CT scan data, and results are transmitted via HeartFlow’s secure Web interface as an interactive report.
2. Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI). Guidelines on Myocardial Revascularization. Eur Heart J 2010; 31: 2501-255.


 January 05, 2026
January 05, 2026 









