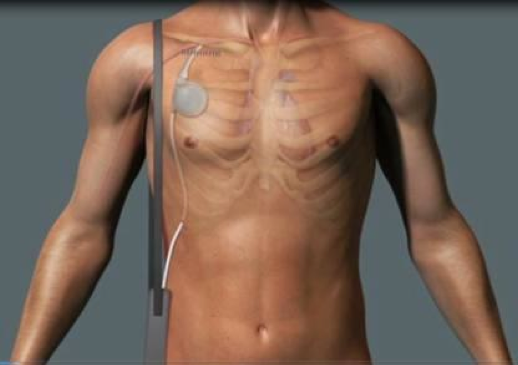
Symphony.
February 2, 2012 — Abiomed Inc. announced that initial clinical data from the safety study for Symphony was presented by Renzo Cecere, M.D., FRCSC, FACS, associate professor of surgery at McGill University, during the late-breaking clinical trials session at the Society of Thoracic Surgeons (STS) 2012 meeting in Fort Lauderdale, Fla.
The presentation, “Initial Safety Trial of an Implantable, Synchronous, Partial Circulatory Support Device: Symphony,” discussed the initial clinical data from the Symphony safety trial being conducted outside of the United States, and was presented during one of the four late-breaking clinical trials sessions at the STS meeting.
The presentation included analysis of the initial first-in-man experience, originally announced in December 2011, which demonstrated feasibility of the synchronized, implantable heart pump Symphony. The analysis indicated a marked improvement in cardiac output, improved renal function, short intensive care unit (ICU) stay and early patient mobility post implant.
“The concept of a minimally invasive implantable pump for patients in chronic heart failure, coupled with the ability to remodel the heart, is unique and groundbreaking and we are very pleased with the initial findings of Symphony,” said Cecere.
Symphony is designed to treat approximately 90,000 of the 1.6 million New York Heart Association (NYHA) Class III chronic heart failure patients by improving patient hemodynamics and potentially quality of life. The device is designed with the primary goal of lowering hospital costs by stabilizing the progression of heart failure and/or recovering/remodeling the heart.
Symphony is not currently approved for use in the United States.
For more information: www.abiomed.com


 January 05, 2026
January 05, 2026 









