June 6, 2013 — Boston Scientific Corporation reported interim data from the REDUCE-HTN clinical program, which demonstrated a significant and sustained reduction in the blood pressure of patients treated with the Vessix Renal Denervation System. Data on the first 41 subjects enrolled in the REDUCE-HTN clinical program were presented today at the annual EuroPCR Scientific Program in Paris by Joachim Schofer, M.D., of the Hamburg University Cardiovascular Center.
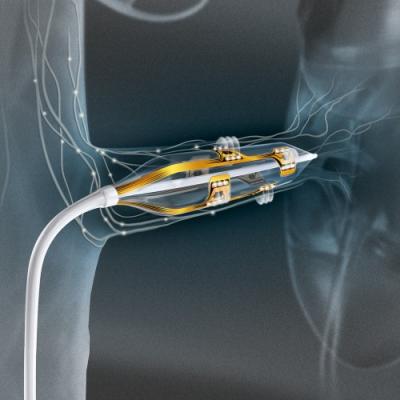
Renal denervation with the Vessix System is a minimally-invasive procedure in which a balloon catheter is fed into the arterial vascular system and positioned in the renal arteries, the major blood vessels that lead to the kidneys. The physician then delivers low-power radiofrequency (RF) energy to disrupt the nerves surrounding the renal arteries in which hyperactivity leads to uncontrolled high blood pressure.
"Tens of thousands of patients worldwide have blood pressure that is high and uncontrolled, putting them at significantly increased risk for a variety of cardiovascular diseases, including heart attack, stroke and kidney failure," said Professor Horst Sievert, M.D., Ph.D., director of the CardioVascular Center Frankfurt, Sankt Katharinen Hospital, in Frankfurt, Germany, and principal investigator in the REDUCE-HTN clinical program. "The interim data from REDUCE-HTN demonstrate that renal denervation with the Vessix System offers a significant and sustained blood pressure reduction in patients with uncontrolled hypertension. The familiar over-the-wire balloon platform of the Vessix System, the short procedure time and precise bipolar approach offer a significant step forward in the treatment of this challenging condition."
The REDUCE-HTN clinical program is evaluating the ability of the Vessix System to reduce blood pressure at six months as compared to the pre-treatment baseline blood pressure. Patients enrolled in the program are required to have a systolic blood pressure of at least 160 mmHg despite taking three or more antihypertensive medications.
Interim data highlights include:
- All patients underwent successful bilateral renal denervation treatment
- At the primary endpoint of six months, patients experienced a significant 27.6 mmHg reduction in systolic blood pressure (p<0.0001)
- No device-related adverse events or procedural renal artery complications were reported
- Long term efficacy was demonstrated, with a sustained 28.4 mmHg reduction in systolic blood pressure in the subset of patients for whom 12-month data are available
- Demonstrated success in treating various anatomies, including accessory renal arteries
- Demonstrated short renal denervation procedural time
"We believe that the familiar balloon catheter design of the Vessix System, intuitive user interface and unique bipolar technology make it the ideal choice for physicians," said Jeff Mirviss, president, Peripheral Interventions, Boston Scientific. "The interim REDUCE-HTN data support the expected long-term benefit of the Vessix System in reducing blood pressure, and we're excited to bring this advanced technology to physicians and their patients."
The Vessix System is a highly-differentiated, advanced renal denervation system that features an intuitive push-button interface, a short 30-second treatment time and an over-the-wire, balloon-based approach familiar to most cardiac and vascular specialists. The Vessix System has both CE Mark and TGA approval and is immediately available for sale in Europe, the Middle East, Australia, New Zealand and select markets in Asia. The Vessix System is not approved for use or sale in the United States.
For more information: www.bostonscientific.com


 January 05, 2026
January 05, 2026 









