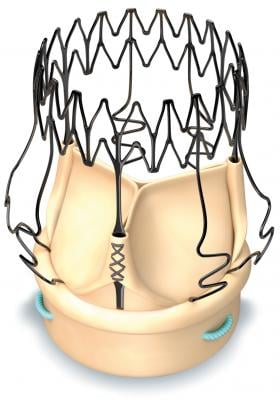
March 30, 2018 — LivaNova PLC announced the first patient enrollment in the Perceval Valve Clinical study for Chinese Registration (“PERFECT”) Trial. The study is a pre-market, prospective, single-arm trial. The PERFECT trial is being conducted to demonstrate the safety and effectiveness of the Perceval sutureless aortic heart valve when used to replace a diseased native or malfunctioning prosthetic aortic valve in the indicated Chinese population for tissue heart valve replacement.
The PERFECT trial is expected to enroll approximately 160 patients who will receive five years of follow-up at eight investigational sites in China. The primary endpoint of the trial is a one-year composite endpoint of major cardiac events as per the Clinical Events Committee adjudication.
Perceval is specifically designed to reduce the physiological impact of surgical aortic valve replacement, simplify complex and minimally invasive procedures, and improve patient outcomes.
“We greatly look forward to the possibility of making this innovative technology, which has demonstrated excellent outcomes in Europe and the United States, available to patients in China,” said Prof. Shengshou Hu, principal investigator and president of Fu Wai Hospital, Chinese Academy of Medical Sciences, Beijing, China. “The excellent hemodynamic characteristics of the Perceval valve, along with its suitability for less invasive approaches, makes introduction of this valve to the greater Chinese population particularly appealing.”
For more information: www.livanova.com


 January 05, 2026
January 05, 2026 









