October 18, 2023 — A study published in The Annals of Thoracic Surgery demonstrates outstanding long-term survival following low-risk isolated surgical aortic valve replacement (SAVR).
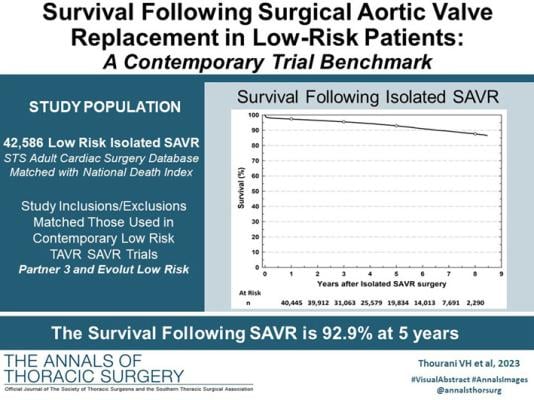
The study reviewed 42,586 patients who underwent low-risk isolated SAVR between 2011 and 2019 at 981 different cardiac surgery programs across the U.S. Conducted by eight leading national investigators, the assessment relied on evidence from The Society of Thoracic Surgeons (STS) National Database, with linkage to the Centers for Disease Control and Prevention’s National Death Index (NDI).
The STS National Database, with nearly 10 million patient records, is the nation’s largest and most comprehensive clinical registry for cardiothoracic disease. The linkage to the NDI has permitted highly accurate survival information to now be available following heart and lung operations performed in the U.S.
All patients in the study were classified as low risk for SAVR mortality as defined by the STS predicted risk of operative mortality (PROM ≤ 4%). Overall, 92.9% survived after five years, and the eight-year survival was close to 90%. The study matched the criterion used in the current low-risk trials evaluating SAVR with transcatheter aortic valve replacement (TAVR). The average age of patients was 74.2 years with a mean PROM of 1.92%
“This study sets a benchmark for outcomes for management of aortic valve stenosis,” said Vinod Thourani, MD, Bernie Marcus Chair of Cardiovascular Surgery and the Marcus Valve Center, Piedmont Heart Institute, Atlanta, Georgia. “This analysis is significant because of the longitudinal follow-up with very large numbers of patients, and it is an important complement to results from randomized clinical trials that are commonly measured over just one year.”
Recent evidence has revealed that SAVR following failed TAVR requiring explant of the TAVR valve, has been the fastest-growing cardiac procedure over the last five years, associated with worse outcomes than those expected following primary SAVR surgeries.
The most recent mid-term results from the PARTNER 3 and Evolut Low-Risk trials comparing risk of death and stroke from SAVR and TAVR are expected to be released in fall 2023. As there have been some concerns raised regarding the representativeness of these trials, the current study was performed to establish real-world long-term results from SAVR to serve as a benchmark for outcomes.
“There remains absolutely no doubt that TAVR is an excellent primary option for patients of higher risk or advanced age. The aim of this study was to specifically evaluate patients of low risk, and this revealed that longitudinal outcomes of SAVR in this specific population are outstanding. As surgeons are consistently performing these surgeries with excellent outcomes, and now in minimally invasive fashion, these data should give providers of valve therapy, including cardiologists and surgeons, a point of reflection when it comes to deciding what the best option is for their patients,” said senior study author Vinay Badhwar, MD, Professor and Chairman of the Department of Cardiovascular and Thoracic Surgery of West Virginia University, and Executive Chair of the WVU Heart and Vascular Institute.
The STS National Database includes 97% of all cardiac operations performed in the U.S. with precise and validated statistical models for patients receiving adult cardiac, general thoracic, and congenital operations.
For more information: https://www.sts.org/
Related content:
Data from Benchmark Registry Demonstrate Improved TAVR Efficiency with Preserved Patient Safety
CRF Announces TCT 2023 Late-breaking Clinical Trials
Edwards Sapien 3 TAVI Granted European Approval to Treat Low-risk Patients
National Coverage Determination Will Make TAVR Available to More Patients at More Centers
VIDEO: TAVR Stands Equal to Surgical Valve Replacement — Interview with Michael Reardon, M.D.
Study Finds TAVR Can Be Considered for Patients in All Surgical Risk Classes
VIDEO: Edwards Lifesciences Sapien 3 TAVR Procedural Animation
TAVR Performs Well Compared to Surgical Aortic Valve Replacement in Low Risk Patients
CMS Finalizes Updates to Coverage Policy for Transcatheter Aortic Valve Replacement
TAVR Outperforms Surgery in Younger, Low-Risk Aortic Stenosis Patients
VIDEO: Tracking Transcatheter Valve Outcomes in the STS-ACC TVT Registry — interview with John Carroll, M.D.
Interventional Imagers: The Conductors of the Heart Team Orchestra
Boston Scientific Receives FDA Approval for Lotus Edge Aortic Valve System
Length of Stay Impacts TAVR Outcomes
VIDEO: TAVR For Asymptomatic Severe Aortic Stenosis — Interview with Philippe Genereux, M.D.
How to Perform Transcaval TAVR Access
The Essentials of Structural Heart Imaging
Hospital Consolidation May Increase Access to TAVR, New Cardiac Technologies
VIDEO: Outcomes Following Urgent TAVR - Results from the TVT Registry — Interview with Sammy Elmariah, M.D.
Valve Replacement Volume Key to Successful Patient Outcomes
TAVR Operator and Hospital Requirements Outlined in 2018 AATS/ACC/SCAI/STS Expert Consensus
VIDEO: Overview of University of Colorado Structural Heart Program
VIDEO: TAVR for Degenerated Surgical Valves, Valve-in-Valve TAVR Procedures — Interview with Sammy Elmariah, M.D.
VIDEO: Advice For Hospitals Starting a Structural Heart Program — interview with John Carroll, M.D.
VIDEO: How to Get Referral Patients Into a Structural Heart Program


 January 05, 2026
January 05, 2026 









