July 12, 2018 — Intact Vascular Inc. announced the publication of the iDissection Classification trial results in the current issue of Journal of Invasive Cardiology.[1]
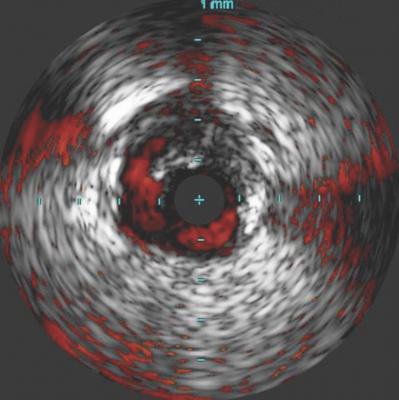
Post-percutaneous transluminal angioplasty (PTA) dissections are often overlooked, underdiagnosed and left untreated. These dissections can compromise clinical outcomes in both the short and long term. The use of intravascular ultrasound (IVUS) has been found to help visualize the presence and severity of dissections not typically seen on angiography.
In the iDissection study, 15 patients with femoropopliteal disease were treated with atherectomy and adjunctive PTA. Angiographic and IVUS images utilizing ChromaFlo Imaging (Philips) were obtained at baseline, post-atherectomy and post-angioplasty, and evaluated for the presence and severity of dissections. Dissections seen on angiography were graded per the NHLBI scale, and IVUS images were graded using the iDissection classification. All images were independently adjudicated by multiple core laboratories. While angiography identified eight dissections, IVUS revealed 46 dissections — a ratio of 6:1. The iDissection study reveals that significant dissections may not be thoroughly observed with angiography yet are substantially more visible when using IVUS, potentially altering the course of patient treatment in real-time.
“Angiography is a suboptimal test to visualize the peripheral arteries. It underestimates vessel size, the presence and extent of calcium, thrombus and stenosis, and does not give a clear picture of optimal stent expansion and apposition. Moreover, the iDissection data confirms that angiography seriously underestimates the presence and severity of dissections following endovascular intervention,” commented Nicolas W. Shammas, M.D., founder and research director, Midwest Cardiovascular Research Foundation, Davenport, Iowa. “The iDissection study validates the need for more sophisticated imaging, such as IVUS, to evaluate acute procedural results.”
Intact Vascular is sponsoring three clinical trials to evaluate its Tack Endovascular System: TOBA II, TOBA II BTK and TOBA III. TOBA II is investigating the combination of the Tack implant with both plain angioplasty balloons and the Bard Lutonix drug-coated balloon (DCB) in the arteries above the knee, and completed enrollment in March 2017. TOBA II BTK is investigating the combination of the Tack implant with plain balloon angioplasty in the arteries below the knee and is actively enrolling patients. TOBA III has completed enrollment in Europe and is investigating the combination of the Tack implant with the Medtronic In.Pact Admiral (DCB), inclusive of long lesions.
For more information: www.intactvascular.com
Reference:


 January 05, 2026
January 05, 2026 









