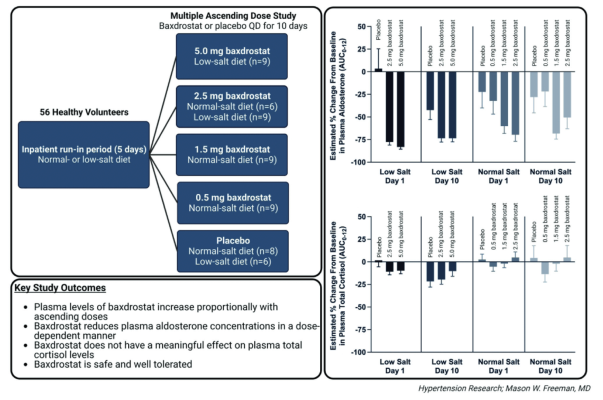
October 24, 2022 — CinCor Pharma, Inc. announced the publication of Phase 1 clinical data for baxdrostat, a highly selective, once daily, oral small molecule inhibitor of aldosterone synthase, in the journal Hypertension Research.
The publication includes clinical data from the company’s randomized, Phase 1, placebo controlled multiple ascending dose (MAD) study evaluating the safety, pharmacokinetics, and pharmacodynamics of baxdrostat in healthy volunteers. These data demonstrate baxdrostat is well tolerated with a half-life that supports once-daily oral dosing. The dose-dependent reduction in plasma aldosterone and lack of impact on cortisol reinforce the selective blockade of aldosterone synthase by baxdrostat.
“We believe these proof-of-concept data support further clinical advancement of this promising new treatment for hypertension,” said Mason Freeman, M.D., Chief Medical Officer at CinCor. “Importantly, these data provide the first clinical evidence that an aldosterone synthase inhibitor can block aldosterone production without impacting cortisol and support a well-tolerated and well-behaved pharmacokinetic profile across all doses of baxdrostat tested.”
The full manuscript, titled “Results from a phase 1, randomized, double-blind, multiple ascending dose study characterizing the pharmacokinetics and demonstrating the safety and selectivity of the aldosterone synthase inhibitor baxdrostat in healthy volunteers” can be found at the following link: https://rdcu.be/cXXng.
For more information: https://www.cincor.com/


 January 05, 2026
January 05, 2026 









