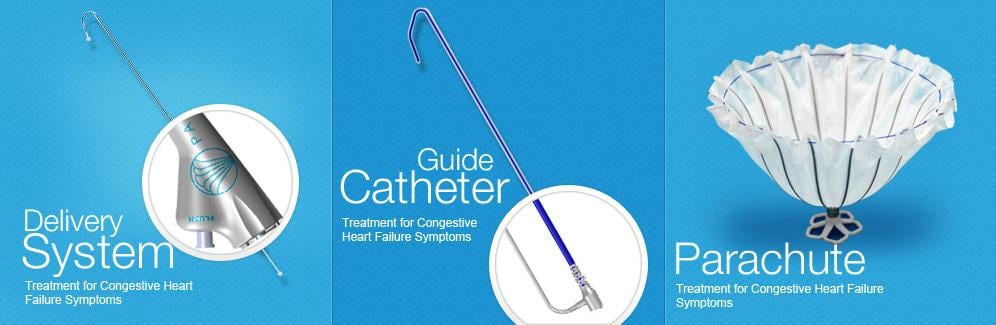
Image courtesy of CardioKinetix Inc.
January 23, 2015 — CardioKinetix Inc. announced the completion of enrollment in Parachute China, a multi-center trial to evaluate the minimally invasive Parachute Ventricular Partitioning Device for the treatment of heart failure.
Results of Parachute China will be presented at the China Interventional Therapeutics (CIT) 2015 conference, which will take place March 19-22 in Beijing, China.
Parachute China enrolled NYHA II to ambulatory IV ischemic heart failure patients with ejection fraction 15-40 percent to support the regulatory submission for the Parachute device to the China Food and Drug Administration (CFDA). The primary endpoint of the study is reduction in left ventricle end systolic volume index (LVESVi) at three months, which will be analyzed at an echocardiography core lab, Yale Cardiovascular Research Group.
The principal investigator is Gao Runlin, M.D, chief cardiologist, Fu Wai Hospital, Chinese Academy of Medical Sciences. Huo Yong, M.D., director of the Heart Center and Department of Cardiology PKU-1st Hospital, and president, Chinese Society of Cardiology and Yang Yuejin, M.D., Ph.D., vice president of Cardiovascular Institute and Fu Wai Hospital serve as co-principal investigators.
After a heart attack, many patients experience enlargement of their left ventricle causing a decrease in cardiac output, which results in heart failure symptoms such as shortness of breath. The Parachute device offers the first minimally invasive catheter-based treatment to partition the damaged muscle, excluding the non-functional heart segment from the healthy, functional segment to decrease the overall volume of the left ventricle and restore its geometry and function. The procedure is performed in the catheterization laboratory under limited sedation. Clinical data demonstrates improved overall cardiac function and quality of life for patients treated with the Parachute device.
The Parachute Ventricular Partitioning Device received CE Mark in 2011. In the United States the Parachute system is an investigational device limited by federal law to investigational use only and is not available for sale.
For more information: www.cardiokinetix.com


 January 05, 2026
January 05, 2026 









