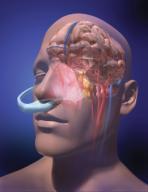
February 5, 2009 - BeneChill Inc. this week started the PRINCE (Pre-Resuscitation Intra-Nasal Cooling Effectiveness) study, a randomized study to determine whether intra-nasal cooling using the RhinoChill device during resuscitation increases patient and survival rates, including ischemic events such as cardiac arrest and stroke.
The PRINCE study is being conducted as a post-market approval study in Europe. The company received CE marking for the RhinoChill system in 2007. The noninvasive, portable device is used without external power for rapid therapeutic patient cooling. This system uses a nasal catheter to deliver a proprietary inert coolant to the nasal cavity to reduce temperature in clinically indicated conditions. Unlike other cooling devices, the RhinoChill device is easily utilized in emergency field settings.
As part of the upcoming study, patients will receive either advanced cardiac life support measures (ACLS) alone or ACLS supplemented by intra-nasal cooling. The study will be conducted in Belgium, Germany, Italy and Sweden over the next six months.
The maker said out-of-hospital cardiac arrest is a significant cause of death, and mild hypothermia induced after resuscitation from cardiac arrest has been shown to improve survival in humans. The device is inserted into the nasal cavity, which has both easy access, has close proximity to the brain, and is a natural heat exchanger for the body.
For more information: www.benechill.com


 January 05, 2026
January 05, 2026 









