December 22, 2011 – Abbott today announced the initiation of ESPRIT I, a first-of-its-kind clinical trial in Europe ...
Stents Peripheral
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
November 4, 2011 — Medtronic Inc. announced approval by the U.S. Food and Drug Administration (FDA) of the Assurant ...
November 1, 2011 – Boston Scientific released the schedule of its major events and product-related clinical research for ...

October 14, 2011 – A U.S. Food and Drug Administration (FDA) review panel gave its unanimous recommendation for the ...
October 3, 2011 – Maquet Cardiovascular has signed a definitive agreement to acquire Atrium Medical Corp. for $680 ...

There have been several key news items from the U.S. stent market over the past year. These include the introduction of ...
September 13, 2011 – An U.S. Food and Drug Administration’s (FDA) will discuss recommendations for Cook Medical Zilver ...
August 10, 2011 – The U.S. Food and Drug Administration (FDA) has approved Abbott’s RX Herculink Elite Renal Stent ...
August 2, 2011 – The U.S. Food and Drug Administration said it recently cleared Abbott Vascular’s RX Herculink Elite ...
July 20, 2011 — IDEV Technologies Inc. announced the completion of enrollment in the SUPERB trial, a U.S. Food and Drug ...
July 18, 2011 — NSVascular Inc., a newly formed subsidiary of NeuroSigma Inc., has signed an exclusive license with the ...
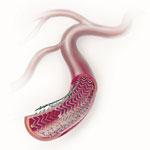
The goal of treating peripheral artery disease (PAD) in the superficial femoral artery (SFA) is limb salvage and ...
June 17, 2011 — The U.S. Food and Drug Administration (FDA) said Boston Scientific has issued a class I recall for its ...
June 3, 2011 - IDEV Technologies announced that data from a long lesion study of 182 patients with significantly ...
May 10, 2011 – Patients suffering from blockages of the arteries to the kidneys now have access to a new stenting ...


 December 22, 2011
December 22, 2011









