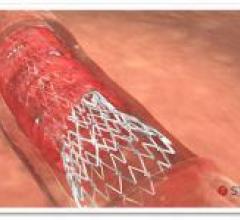
February 8, 2016 — Stentys announced the first distribution agreements for its drug-eluting stent for treating below-the ...
Stents Peripheral
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
January 29, 2016 — Intact Vascular Inc. announced that positive six-month results from its Tack Optimized Balloon ...
December 31, 2015 - The U.S. Food and Drug Administration (FDA) has cleared Biotronik's Astron Peripheral Self-Expanding ...
October 1, 2015 — Kimihiko Kichikawa, M.D., department of radiology at Nara Medical University in Japan, reported two ...
October 1, 2015 — Stentys announced that the company’s drug-eluting stent received CE Mark for treatment of below-the ...
September 28, 2015 — New 12-month clinical trial outcomes assessing the safety and performance of the Boston Scientific ...
August 28, 2015 — PinnacleHealth CardioVascular Institute enrolled the first patient in Pennsylvania and second in the ...
August 20, 2015 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) approval for the Innova ...
July 17, 2015 - Veryan Medical announced that the first subject has been enrolled in their MIMICS-2 study at ...
July 16, 2015 — Medtronic plc announced that its Protege GPS self-expanding peripheral stent system has received ...
June 10, 2015 - Biotronik announced the publication of promising clinical results regarding its Pulsar-18 peripheral ...
April 29, 2015 — A key trial evaluating the Boston Scientific Eluvia drug-eluting vascular stent system met its primary ...
March 9, 2015 — Boston Scientific Corp. announced the settlement of the breach of merger agreement lawsuit brought by ...
February 23, 2015 – Biotronik announced the presentation of data from the iliac arm of the BIOFLEX-I clinical trial at ...

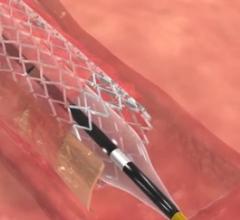
 February 08, 2016
February 08, 2016