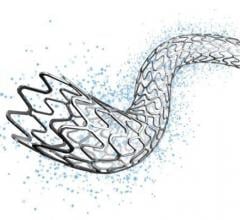
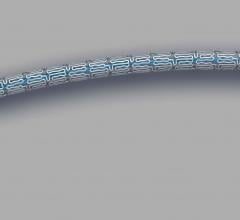
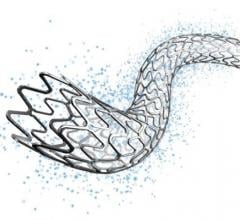
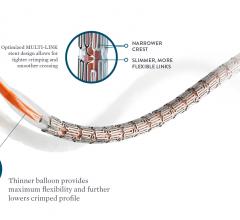
May 25, 2018 — Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for Xience Sierra ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
DAIC Editor Dave Fornell takes a tour of some of the most interesting new technologies on the expo floor at the 2018 ...
February 26, 2018 – Designed specifically for small vessels, Medtronic plc announced U.S. Food and Drug Administration ...
February 19, 2018 — The Detroit Medical Center’s (DMC) interventional cardiology team at Heart Hospital recently became ...
February 13, 2018 — Abbott announced the first patient has been enrolled in a clinical trial evaluating 28 days of dual ...
February 1, 2018 – Thomas Zeller (Bad Krozingen, Germany) presented the 12-month results from Veryan Medical’s MIMICS-2 ...
January 25, 2018 – Elixir Medical Corporation, a leader in the development of breakthrough adaptive remodeling ...
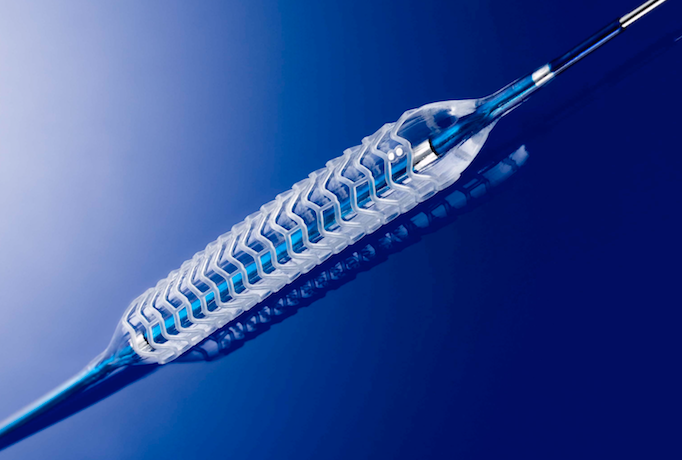
There was no shortage of interest in bioresorbable stent technologies at the many sessions offered on this topic or ...
December 13, 2017 — Cordis, a Cardinal Health company, and Medinol recently announced U.S. Food and Drug Administration ...
November 24, 2017 – During a late-breaking session at the European Society of Cardiology (ESC) 2017 meeting, presented ...
November 10, 2017 — Cordis, a Cardinal Health company, recently unveiled a comprehensive interventional cardiology portf ...
November 9, 2017 — Abbott received European CE mark for Xience Sierra, the newest generation of the company's Xience ...
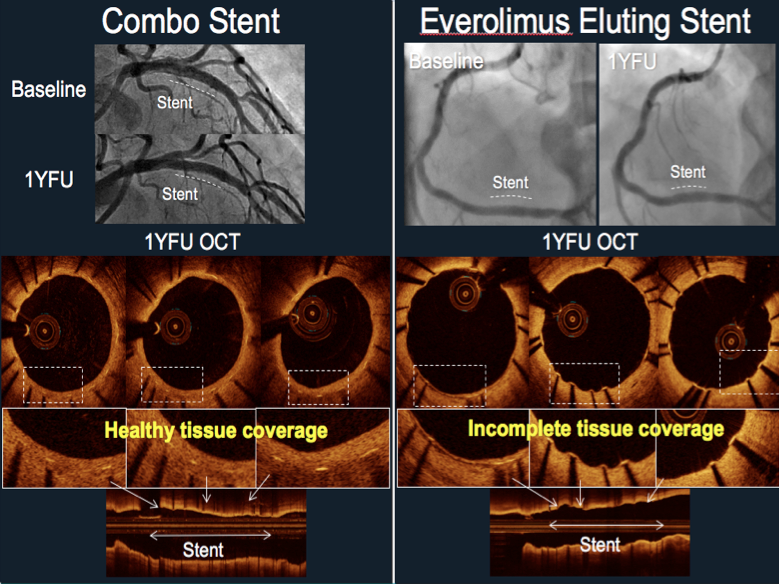
November 8, 2017 – New results from the HARMONEE Japan/U.S. Registration Trial, reported by in a first report ...
November 6, 2017 — The first trial to evaluate the safety of dual antiplatelet therapy (DAPT) for less than 12 months ...

 May 25, 2018
May 25, 2018