Dr. Stephen Worthley, Ph.D., director of the cardiac cath labs at Royal Adelaide Hospital at the University of Adelaide ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
Dean Kereiakes, M.D., medical director of The Christ Hospital Heart and Vascular Center, and a lead investigator for ...
October 27, 2015 — Results from the multicenter, prospective, randomized PANDA III trial indicate that the BuMA sirolimu ...
October 26, 2015 — OrbusNeich announced the presentation of the one-year clinical outcomes from the 1,000-patient REMEDE ...
October 23, 2015 — Results of the TUXEDO trial, designed to compare two types of stents in diabetic patients, found ...
October 22, 2015 — Results from the ISAR-DESIRE 4 trial indicate that use of a scoring balloon plus a paclitaxel-coated ...
There were several overarching technology trends seen at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) annual ...
October 16, 2015 — Enrollment has successfully concluded in its multi-center, international CE mark clinical trials MEND ...
October 5, 2015 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific’s Synergy Bioabsorbable ...
June 17, 2015 - Stentys announced that the findings of the first Xposition clinical experience, as part of the SETUP ...
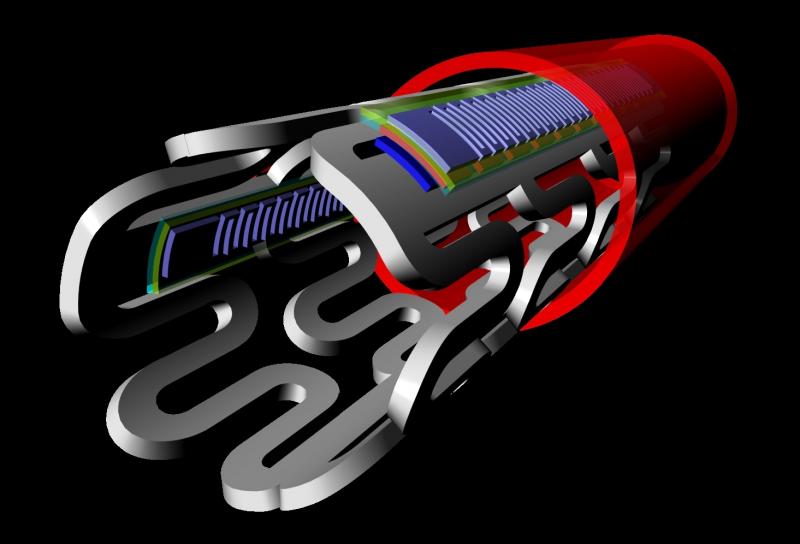
With the recent innovation of flexible microelectronic sensor circuits that can be made from bioresorbable materials, it ...
May 28, 2015 — OrbusNeich has announced that the first U.S. patient has been enrolled in the HARMONEE (Harmonized ...
May 22, 2015 — Boston Scientific reported positive, long-term data from the EVOLVE Trial of the Synergy everolimus ...
May 11, 2015 — Boston Scientific Corp. presented an overview of its continued business momentum and long-term growth ...
March 25, 2015 — Despite the advent of a new generation of stents, patients with multiple narrowed arteries in the heart ...


 October 30, 2015
October 30, 2015