July 5, 2022 — A BIO-RESORT subgroup analysis of outcomes in small coronary vessels (<2,5mm) evaluated the efficacy and ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
June 2, 2022 — Elixir Medical, a developer of innovative, drug-eluting cardiovascular devices, announced print ...
May 20, 2022 — Results from a real-world study investigating safety and effectiveness of clopidogrel versus aspirin ...
May 20, 2022 — New long-term data from the Safety Assessment of Femoropopliteal Endovascular Treatment With PAclitaxel ...
February 16, 2022 – Elixir Medical, a developer of innovative, drug-eluting cardiovascular devices, has announced ...
November 9, 2021 — Results from SUGAR trial, a randomized, controlled, multicenter trial conducted exclusively in ...
October 20, 2021 — Boston Scientific Corporation announced positive data for the Eluvia Drug-Eluting Vascular Stent ...
September 1, 2021 — The STOPDAPT-2 ACS trial does not support the use of one month of dual antiplatelet therapy (DAPT) ...
June 30, 2021 — Abbott announced its Xience family of drug-eluting coronary stents received U.S. Food and Drug ...
June 7, 2021 — A couple years ago a study showed a mortality safety signal in patients who underwent peripheral artery ...
April, 14, 2021 – Elixir Medical recently announced the first patient was treated in the BIOADAPTOR randomized ...
April 6, 2021 — Abbott today announced its Xience stent has received CE mark in Europe for shorter duration of dual anti ...
January 26, 2021 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific's Synergy Megatron Drug ...
Here are some of the key takeaways from the late-breaking interventional cardiology and structural heart trials ...

October 17, 2020 – In a surprise to many, a randomized clinical trial found that drug-eluting stents (DES) with durable ...


 July 05, 2022
July 05, 2022







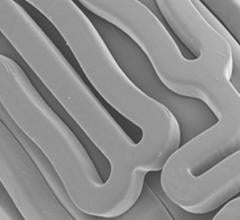


![Comparison showing platelet adhesion to the surface of various coronary artery drug-eluting stents (DES) in a preclinical study that used aspirin only. Abbott said the Xience stent's fluoropolymer is significantly more anti-thrombotic than other DES.[2]](/sites/default/files/styles/content_feed_medium/public/DES_Comparison_thrombus_formation_Stents_Abbott.jpg?itok=mfh9GUz-)
