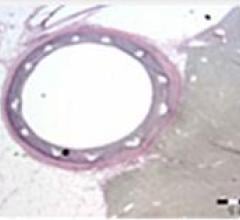
October 5, 2015 — The U.S. Food and Drug Administration (FDA) has approved Boston Scientific’s Synergy Bioabsorbable ...
Stents Bioresorbable
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents

September 4, 2015 — A drug-eluting coronary stent made of absorbable material performed similarly to gold-standard metal ...
September 1, 2015 — A bioresorbable drug-eluting coronary stent showed similar efficacy and safety results compared to a ...
August 26, 2014 — The Cardiovascular Research Foundation (CRF) announced the late-breaking trials and first report ...
August 5, 2015 — The global market value for bioabsorbable scaffolds (BAS) will expand rapidly from $143.7 million in ...

July 20, 2015 - Boston Scientific has initiated a study of the company's first fully resorbable drug-eluting scaffold ...
June 5, 2015 - REVA Medical Inc. announced the initiation of patient enrollment in the FANTOM II clinical trial of its ...
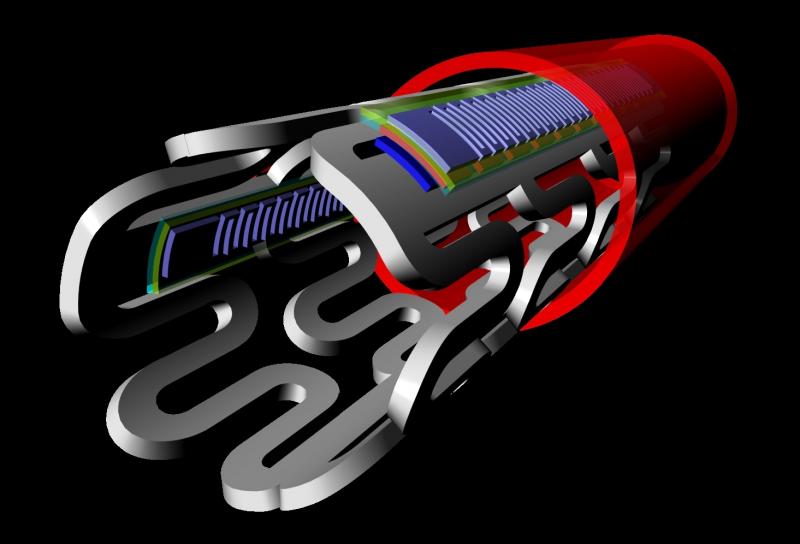
With the recent innovation of flexible microelectronic sensor circuits that can be made from bioresorbable materials, it ...

May 26, 2015 — Privately-held company Arterial Remodeling Technologies (ART) announced CE Mark clearance for its next ...
May 22, 2015 — Boston Scientific reported positive, long-term data from the EVOLVE Trial of the Synergy everolimus ...
May 18, 2015 — Amaranth Medical announced plans to report clinical results from the company's ongoing trial of its ...
May 19, 2015 — Abbott announced that it has received CE Mark for the latest advancement of its Absorb stent system ...
May 11, 2015 — Boston Scientific Corp. presented an overview of its continued business momentum and long-term growth ...
February 19, 2015 — Biotronik announced the completion of enrollment in the BIOSOLVE-II trial — a clinical study investi ...
February 17, 2015 — Micell Technologies, Inc. announced the commercial availability of the MiStent sirolimus-eluting abs ...


 October 05, 2015
October 05, 2015