October 28, 2016 — Svelte Medical Systems Inc. announced this week it received CE Mark certification of the Direct ...
Stents Bioresorbable
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents
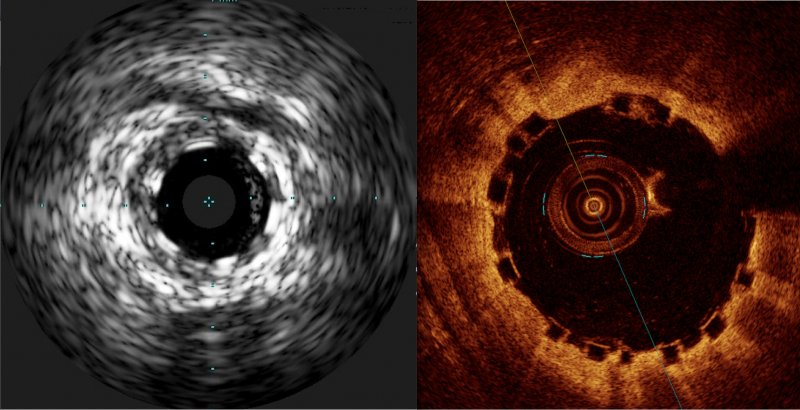
There has been a lot of interest in the interventional community regarding the Abbott Absorb Bioresorbable Vascular ...

September 28, 2016 — The Cardiovascular Research Foundation (CRF) included 11 late-breaking trials and 16 first report ...
There was an explosion of interest in bioresorbable stents immediately following the July 5, 2016 announcement that the ...

August 5, 2016 — Here are the top 20 most popular current content on the Diagnostic and Interventional Cardiology ...
July 26, 2016 — On July 20, Massoud Leesar, M.D., of University of Alabama at Birmingham Hospital implanted a patient ...
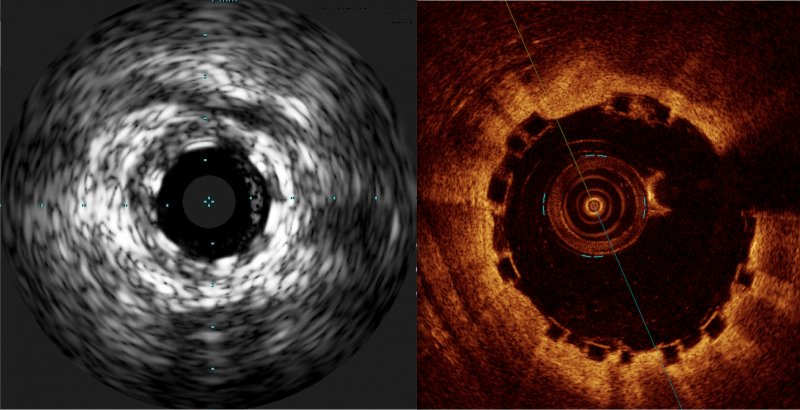
Some have labeled bioresorbable scaffolds (BRS), also known as bioresorbable stents, as the fourth evolution of ...
July 19, 2016 — Good Samaritan Hospital, Los Angeles, is the first hospital on the West Coast to offer patients with ...
July 11, 2016 — University Hospitals Case Medical Center, Cleveland, is among the first in the country – and the first ...
July 5, 2016 — The U.S. Food and Drug Administration (FDA) has cleared the first fully bioresorbable coronary stent for ...
June 6, 2016 — Amaranth Medical announced that it completed enrollment in May in the RENASCENT-II study of its novel ...
May 17, 2016 — Biotronik presented 12-month data from the BIOSOLVE-II trial during a Hotline Session at EuroPCR 2016 ...

May 16, 2016 — Cardinal Health announced it has entered into a distribution agreement with Biosensors that enables ...

Bioresorbable stents have been one of the hottest new cardiovascular technologies discussed at cardiology meetings over ...
Gregg Stone, M.D., director of cardiovascular research and education at Columbia University Medical Center / New York ...

 October 28, 2016
October 28, 2016