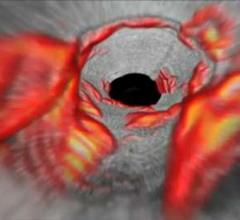
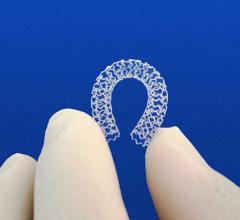
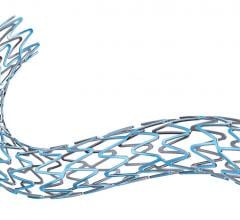
March 15, 2016 — An independent panel of experts convened by the U.S. Food and Drug Administration (FDA) voted 9 to 0 ...
Stents Bioresorbable
This channel includes news and new technology innovations for bioresorbable stents (BRS). These devices are also referred to as bioabsorbable stents, bioresorbable scaffolds and dissolving stents. BRS are designed as an alternative to permanent metallic stent implants, which cause issues in a small number of patients with in-stent restenosis, late-stent thrombosis and require use of long-term antiplatelet therapy. Metallic stents also cause issues with CT and MRI imaging and may prevent future options for coronary bypass graft (CABG) surgery. BRS are supposed to remove avoid these issues by dissolving and disappearing from the vessel after a period of 2-4 years. This, returns the vessel to its natural state and allows for the return of vasodilatation and vasoconstriction. BRS have had some issues in clinical trials not being able to match the performance of standard metallic drug eluting stents (DES) because of their thick stent struts. Newer generation BRS are in development with struts smaller than 100 micros, with will be closer to those of current generation metallic stents
March 11, 2016 — Magnesium Elektron, developer, manufacturer and supplier of high-performance magnesium alloys ...
At the beginning of each year, I always try to determine what the next big cardiovascular technology advances to watch ...
The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel is set to review data and offer ...
For the past few years, the focus of the annual Transcatheter Cardiovascular Therapeutics (TCT) meetings has been on ...
DAIC Editor Dave Fornell offers his choices for the most innovative new interventional cardiovascular technologies ...
Tom Watson, clinical analyst for MDBuyLine, and DAIC Editor Dave Fornell discuss some of the new cardiovascular and ...
October 30, 2015 — Results from a clinical trial showed that that the Abbott Absorb everolimus-eluting bioresorbable ...
Dean Kereiakes, M.D., medical director of The Christ Hospital Heart and Vascular Center, and a lead investigator for ...
October 27, 2015 — Results from the multicenter, prospective, randomized PANDA III trial indicate that the BuMA sirolimu ...
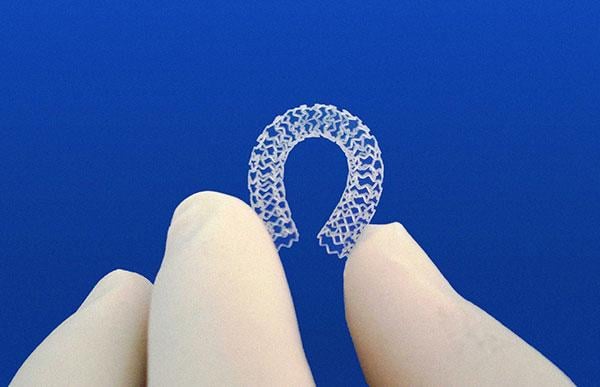
There were several overarching technology trends seen at the 2015 Transcatheter Cardiovascular Therapeutics (TCT) annual ...
October 16, 2015 — Enrollment has successfully concluded in its multi-center, international CE mark clinical trials MEND ...

October 16, 2015 — Results from a clinical trial showed that Abbott's Absorb everolimus-eluting bioresorbable vascular ...
October 15, 2015 — Two new advances in stent technology announced in recent days further reinforce the effectiveness of ...
October 15, 2015 — Biotronik has announced results from the BIOSOLVE-II trial, investigating the safety and clinical ...


 March 15, 2016
March 15, 2016