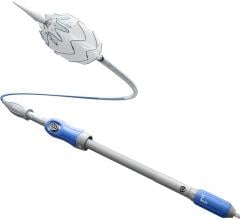
May 15, 2019 — W. L. Gore & Associates Inc. (Gore) announced the U.S. Food and Drug Administration (FDA) has granted ...
Stent Grafts
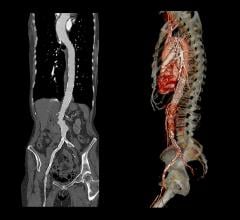
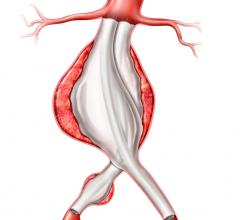
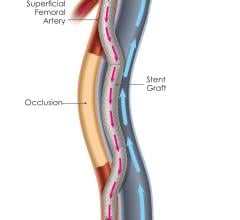
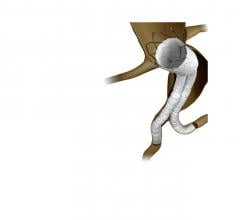
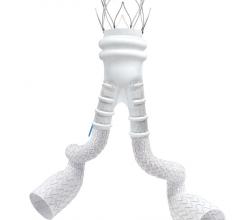
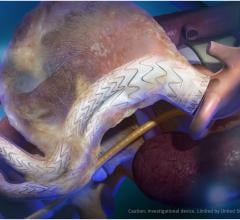
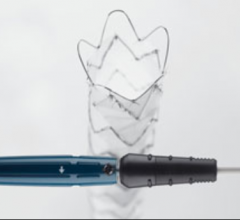
Stent grafts are used in transcatheter endovascular aortic repair (EVAR) procedures to seal aortic aneurysms. The grafts usually consist of a self-expanding stent frame that is covered with material to seal the vessel walls and prevent blood leaks feeding the aneurysm.
April 15, 2019 — Biotronik began its U.S. commercial launch of the PK Papyrus covered coronary stent system for use in ...
February 14, 2019 — Abdominal aortic stent grafts will remain the largest segment in the global aortic stent graft ...
February 7, 2019 — Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for the Valiant ...
January 7, 2019 — Endologix Inc. announced that in order to ensure optimal outcomes for patients, unrestricted sales and ...
October 8, 2018 — Endologix Inc. received notice that the U.S. Food and Drug Administration (FDA) has classified a ...
July 16, 2018 — Endologix Inc. announced one-year results from the LUCY (Evaluation of FemaLes who are Underrepresented ...
July 2, 2018 — The PQ Bypass Detour System showed promising 12-month durability for patients with extremely long ...
June 13, 2018 — Cardinal Health announced that its Incraft Abdominal Aortic Aneurysm (AAA) Stent Graft System has ...
February 6, 2018 – Endologix, a developer and marketer of treatments for aortic disorders, announced the completion of ...

January 8, 2018 — W. L. Gore & Associates Inc. announced the first implant of the Gore Excluder Conformable AAA ...
October 9, 2017 — The U.S. Food and Drug Administration (FDA) is evaluating recent information regarding Type IIIa and ...

September 14, 2017 — Here are quick summaries for all the key late-breaking vascular and endovascular clinical trials ...


 May 15, 2019
May 15, 2019