July 2, 2018 — The PQ Bypass Detour System showed promising 12-month durability for patients with extremely long blockages in the superficial femoral artery (SFA) in new results from the DETOUR I Trial. The data were presented as a late-breaking clinical trial session at the Society for Vascular Surgery (SVS) Vascular Annual Meeting, June 20-23 in Boston, Mass. The trial enrolled lesions longer and more complex than those typically studied in SFA clinical trials, with an average lesion length of 37cm, 100 percent TASC II C/D, 96 percent chronic total occlusions (CTO) and 81 percent with moderate-to-severe calcification.
The DETOUR I trial — a prospective, single-arm, multicenter, core lab-adjudicated study — enrolled and treated 77 patients and 81 lesions. Primary and primary-assisted patency at 12 months in all lesions of the DETOUR I trial was 73 percent and 80 percent, respectively. Secondary patency was achieved in 94 percent of patients. Additional safety and effectiveness outcomes include 100 percent freedom from amputation, 99 percent freedom from acute limb ischemia, and Rutherford improvement of ≥ 2 classes in 90 percent of patients.
“The lesions treated in DETOUR I were more than just long, they were extremely long, completely blocked and severely calcified,” said Dainis Krieviņš, M.D., Ph.D., vascular surgeon and director of the Institute of Research at Pauls Stradins Clinical University Hospital, Riga, Latvia. “Endovascular devices currently approved for use on these incredibly challenging lesions have surprisingly low durability in lesions that are half the size of the average DETOUR lesion and fail approximately 40 percent of the time by the 12-month milestone. This truly highlights the need for the minimally invasive DETOUR procedure for long, complex SFA lesions.”
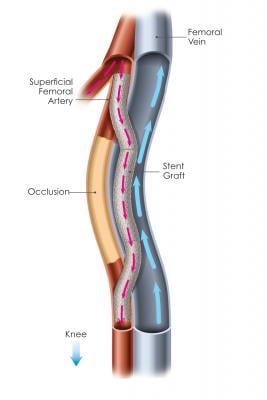
Percutaneous femoropopliteal bypass (the Detour procedure) is an entirely new procedure enabled by PQ Bypass’s proprietary Detour System. The Detour procedure creates a pathway with PQ Bypass’s proprietary stent grafts, originating in the SFA, traveling through the femoral vein and ending in the popliteal artery, bypassing the diseased part of the artery. The stent grafts redirect oxygen-rich blood around the blockage, restoring blood flow to the lower leg and foot of the patient.
For more information: www.pqbypass.com


 January 05, 2026
January 05, 2026 









