June 13, 2018 — Cardinal Health announced that its Incraft Abdominal Aortic Aneurysm (AAA) Stent Graft System has received a favorable recommendation on its premarket approval application from a key U.S. Food and Drug Administration (FDA) panel. The FDA Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 11 to 4 in favor of the benefits of the device.
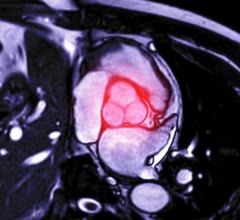
The Incraft system is an advanced endovascular aneurysm repair (EVAR) technology for the treatment of infrarenal abdominal aortic aneurysms (AAAs), a severe and complex condition.
An abdominal aortic aneurysm is a bulging, weakened area in the wall of the lower part of the aorta, the main artery of the body, which, unless treated, can rupture and lead to a life-threatening hemorrhage. An estimated 1.5 million people in the United States have AAA, and more than 200,000 new diagnoses are made each year.1 These aneurysms account for approximately 10,000 deaths annually in the United States.2 Once identified, treatment options for AAAs include medical monitoring, open surgical repair or EVAR, a minimally invasive endovascular treatment option with the potential to reduce perioperative mortality and morbidity.
While several EVAR devices are currently available in the U.S., treatment options are limited for many AAA patients with small femoral or iliac arteries or with heavily calcified or tortuous vessels that could lead to complications during the introduction of EVAR devices.
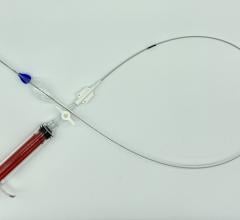
The Incraft system is an ultra-low profile and flexible stent-graft system designed to prevent rupture of infrarenal AAAs in a wide range of patient populations.
The favorable vote of the advisory committee followed a review of clinical data from the pivotal INSPIRATION trial. The prospective, multi-center, single-arm study evaluated the safety and effectiveness of the Incraft system in patients with AAA. The trial showed that the Incraft system met the primary safety and effectiveness endpoints, with a low rate of major adverse events at 30 days and a high rate of successful aneurysm treatment at 1 year.3 As presented at the Circulatory System Devices Panel meeting, the trial demonstrated high survival of nearly 80 percent and no aneurysm ruptures through four years of follow up.
The Incraft system, which received a CE mark in 2014, is commercially available in 39 countries. It is an investigational device not available for sale in the United States.
For more information: www.cardinalhealth.com
References
2. Centers for Disease Control and Prevention (CDC), 2017
3. Ohki, One-Year Outcomes of the INSPIRATION Study of the INCRAFT® Stent-Graft System for Treatment of Abdominal Aortic Aneurysms (AAAs), Society for Vascular Surgery 2015


 September 18, 2025
September 18, 2025