May 17, 2012 — Boston Scientific Corp. announces CE mark and European market launch of the Innova Self-Expanding Bare ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
May 14, 2012 -- Two types of stent systems are both effective in treating patients who have severe forms of peripheral ...
May 4, 2012 - Angioslide Ltd., a provider of embolic capture angioplasty solutions, announced that it has received U.S ...
May 4, 2012 - A new balloon catheter system could advance the endovascular approach to treating obstructed arteries in ...
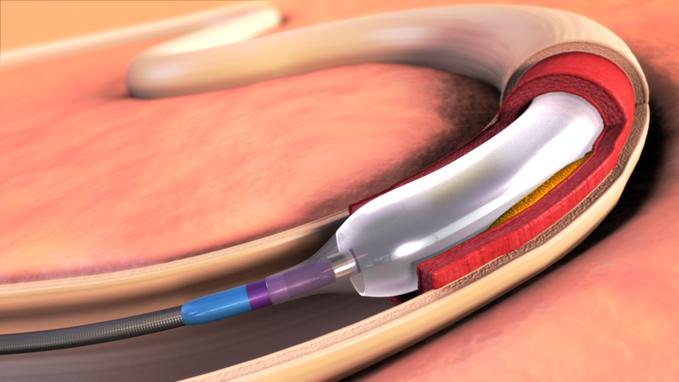
April 17, 2012 –– Expanding its commitment to developing innovative treatments for cardiovascular disease and the ...
March 16, 2012 — The U.S. Food and Drug Administration (FDA) approved the premarket approval (PMA) application for the ...
March 9, 2012 — Avinger Inc. announced the enrollment of the first U.S. patient in the CONNECT II global clinical trial ...
March 6, 2012 — In a development that brings advanced combination therapy treatment of peripheral artery disease (PAD) t ...
March 6, 2012 — Vascular Magnetics announced it has raised $7 million to advance development of a drug delivery system ...
February 9, 2012 — Boston Scientific announced the United States launch of the TruePath CTO device, designed to ...
January 18, 2012 — Boston Scientific reported nine-month clinical endpoint data from its ORION trial, demonstrating ...
January 13, 2012 — Amorcyte Inc.,a NeoStem Inc. company, announced the expansion of intellectual property protection ...
December 22, 2011 – Abbott today announced the initiation of ESPRIT I, a first-of-its-kind clinical trial in Europe ...
December 13, 2011 – Boston Scientific announced the U.S. launch of its Charger PTA Balloon Catheter, a 0.035-inch ...
November 23, 2011 – Vascular occlusions are blocked blood vessels caused by a blood clot in an artery. Blocks in the ...

 May 17, 2012
May 17, 2012








