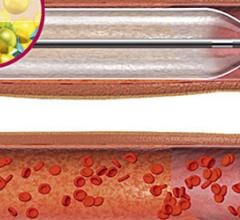
November 1, 2012 — It looks as though drug-eluting balloons (DEBs), already widely used in Europe, are set to burst into ...
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
November 1, 2012 — Terumo Interventional Systems announced the nationwide availability of its new Pinnacle Precision ...
October 26, 2012 — Covidien announced final 12-month results from its DEFINITIVE LE (Determination of Effectiveness of ...
October 24, 2012 — Avinger Inc., a medical device manufacturer of multi-functional catheters for crossing CTOs in ...
October 23, 2012 — The use of stents for arterial blockages in the leg has been associated with high rates of late ...
October 16, 2012 — Proteon Therapeutics Inc. has initiated enrollment in a Phase 1 clinical study of its lead product ...
October 15, 2012 — Three-year data from the Zilver PTX randomized controlled trial of paclitaxel-eluting stents for ...
October 11, 2012 — Spectranetics Corp. announced the U.S. market launch of the Tapas catheter at the 2012 Vascular ...
October 11, 2012 — Boston Scientific Corporation has begun the U.S. and European launch of its Victory guidewire ...
October 8, 2012 — Following Health Canada approval, Cook Medical made the Zilver Vena Venous Self-Expanding Stent availa ...
September 27, 2012 — Boston Scientific has launched the Promus Element Plus BTK (Below The Knee) everolimus-eluting ...
September 20, 2012 — Boston Scientific Corp. has signed a definitive agreement to acquire BridgePoint Medical Inc., a ...
September 18, 2012—Covidien announced the launch of the Viance Crossing Catheter and Enteer Re-entry System for treating ...
August 30, 2012 — According to Millennium Research Group (MRG), an authority on the medical technology market, as ...
August 22, 2012 — Placement of an endovascular stent has become a common procedure for successfully reopening blocked ...

 November 01, 2012
November 01, 2012