This video, provided by Medtronic, demonstrates the implantation of Micra transcatheter pacing system (TPS). The device ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
May 15, 2017 – The Heart Rhythm Society (HRS) in joint partnership with several other electrophysiology societies issued ...
May 15, 2017 — The Heart Rhythm Society (HRS) released a first-of-its-kind consensus statement in the United States on ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 15, 2017 – The U.S. Food and Drug Administration (FDA) has granted market clearance Medtronic’s portfolio of ...
May 12, 2017 — More than one-third of patients undergoing transcatheter aortic valve replacement (TAVR) were observed to ...
May 11, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval of the company's MultiPole Pacing ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
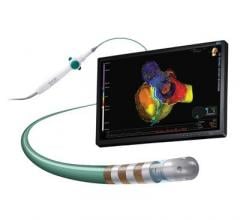
May 10, 2017 — Abbott announced CE Mark of the TactiCath Contact Force Ablation Catheter, Sensor Enabled, developed to ...
May 10, 2017 — Biotronik announced the availability of the first U.S. Food and Drug Administration (FDA)-approved cardi ...
May 9, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and the launch of Sentus ProMRI, the ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
May 9, 2017 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific’s Resonate family of implantable ...
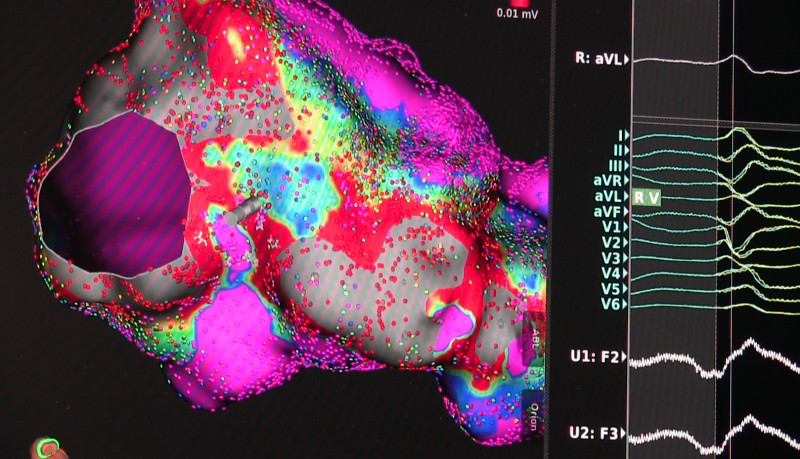
May 9, 2017 — A manuscript by physicians from Mayo Clinic and Harvard Brigham & Women's Hospital entitled, “Initial ...
May 11, 2017 — A new study shows that the Apple Watch's heart rate sensor, when paired with an artificial intelligence ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
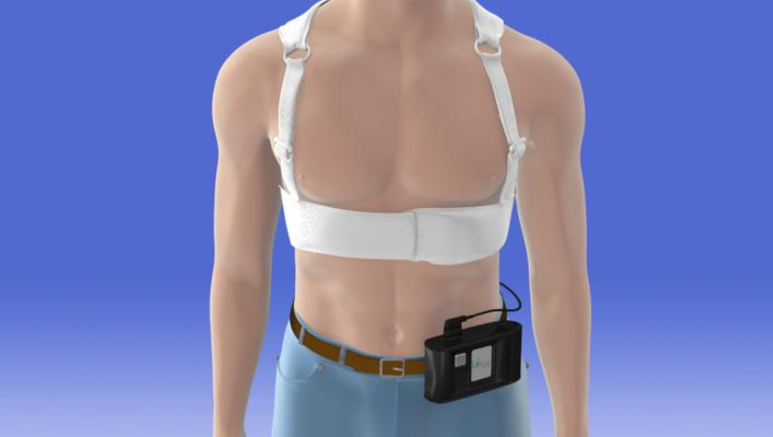
May 11, 2017 — A new study shows that the use of a wearable cardioverter defibrillator (WCD) is safe and effective in ...

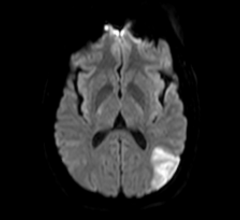
May 12, 2017 – A new study found that dementia rates increase with delays in starting anticoagulation treatment for atri ...
May 12, 2017 – New study proves safety of novel, wireless pacing system, WiSE CRT (Wireless Stimulation Endocardially ...


 May 16, 2017
May 16, 2017