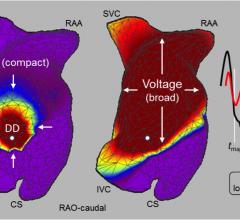
July 19, 2019 — A new method of evaluating irregular heartbeats outperformed the approach that’s currently used widely ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
July 15, 2019 — Acutus Medical announced the publication of the UNCOVER AF study in Circulation: Arrhythmia and ...
July 9, 2019 — South Australian researchers are developing a tiny fiber-optic sensor, which has the potential to change ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
July 5, 2019 — Single-use cardiology medical device reprocessing company Innovative Health, received U.S. Food and Drug ...
July 3, 2019 — The American College of Cardiology (ACC) has partnered with Veradigm, an Allscripts business unit, to ...
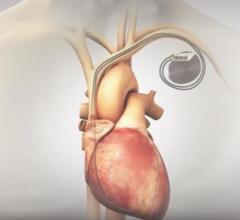
For patients at risk for sudden cardiac arrest (SCA) who are being evaluated for a permanent implantable cardioverter ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
July 1, 2019 — Catheter Precision Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared its new ...
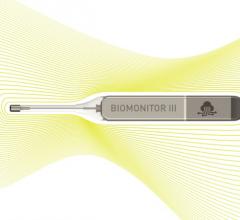
July 1, 2019 – Biotronik announced the European market release of its new injectable cardiac monitor (ICM), Biomonitor ...
Sanjaya Gupta, M.D., electrophysiologist, St. Luke's Mid America Heart Institute, and assistant professor, University of ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
June 17, 2019 – Initial results from the AVIATOR 2 international registry were presented as late-breaking clinical ...
June 14, 2019 — Bardy Diagnostics Inc. announced that its Carnation Ambulatory Monitor (CAM) was recognized with the ...

June 12, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
June 12, 2019 — Boston Scientific Corp. has initiated the OPTION trial to compare safety and effectiveness of the next ...
June 4, 2019 — Orchestra BioMed Inc. announced the presentation of two-year clinical data from the European Moderato I ...
Physicians use many strategies to better interface with patients and their families to try and explain in non-physician ...


 July 19, 2019
July 19, 2019