May 8, 2020 — New clinical trial reveals the first-in-human results for paroxysmal or persistent atrial fibrillation (AF ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.

May 8, 2020 — A new clinical trial is the first to compare the safety and efficacy of subcutaneous implantable ...
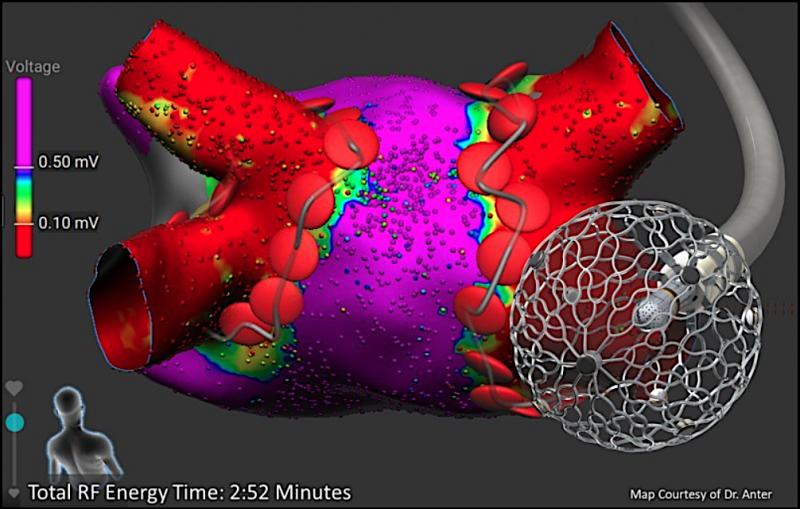
May 8, 2020 — A new clinical trial effectively uses pulsed field (PF) energy to treat patients with persistent or ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
May 2020 — An necklace has been developed that detects abnormal heart rhythm will be showcased for the first time on EHR ...
May 4, 2020 — Cardiac Insight Inc., a U.S.-based digital healthcare innovator specializing in body-worn sensor ...
May 1, 2020 — The Heart Rhythm Society (HRS) has created a COVID-19 Task Force to make recommendations related to ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

April 23, 2020 — The U.S. Food and Drug Administration (FDA) issued a Drug Safety Communication today reminding doctors ...

April 8, 2020 – The scientific community is learning more about the impact and interaction of cardiovascular diseases ...

April 1, 2020 — Due to the continued global escalation of the novel coronavirus (COVID-19, SARS-CoV-2), the Heart Rhythm ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
March 29, 2020 — Patients with atrial fibrillation (AF) who took oral anticoagulants alone after undergoing ...

It has been more than three months since the word coronavirus was uttered on everyone’s lips and all over the social ...
March 20, 2020 — The Centers for Medicare and Medicaid Services (CMS) announced March 18, 2020, that all elective ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
An interview with Ehtisham Mahmud, M.D., FSCAI, chief, Division of Cardiovascular Medicine, executive director of ...

Philips is working on a prototype cath lab angiographic imaging system that might be able to replace the current X-ray ...

Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...


 May 08, 2020
May 08, 2020