July 15, 2019 — Acutus Medical announced the publication of the UNCOVER AF study in Circulation: Arrhythmia and Electrophysiology. The study demonstrated 73 percent single-procedure freedom from atrial fibrillation (AF) at 12 months with the use of Acutus' AcQMap advanced cardiac imaging and mapping system. Acutus develops electrophysiology (EP) technology solutions built into an open platform EP suite of products that enable personalized and adaptive approaches to therapy.
According to the Centers for Disease Control and Prevention, AF increases a person's risk of stroke by five times compared to those without AF. Strokes caused by AF are also more severe than strokes with other underlying causes, indicating the importance of effective treatment. Cardiac ablation is a procedure that can reduce the burden of the arrhythmia and lessen the risk of adverse events, yet traditional ablation procedures often fail to achieve long-term freedom from AF, resulting in repeat ablation procedures.1
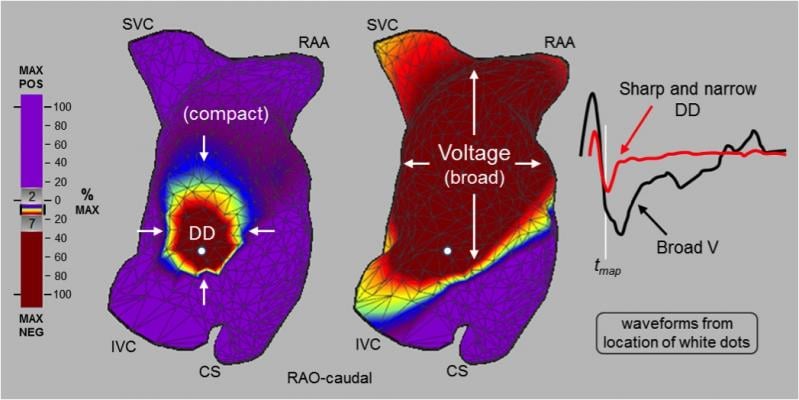
UNCOVER AF prospectively studied the safety and efficacy of non-contact ultrasound imaging and high-resolution charge mapping to identify non-pulmonary vein sources during persistent AF cardiac ablation. One hundred twenty-seven (127) patients at 13 sites in Europe and Canada were treated, 98 percent of which reached sinus rhythm at the end of the procedure. Ninety-three (93) percent of patients achieved freedom from AF after one or two procedures, and 82.4 percent experienced zero episodes of AF greater than 30 seconds during any of the four 24-hour monitoring periods at three, six, nine and 12 months.
"The results from UNCOVER AF have surpassed expectations and shown us the potential to advance outcomes toward AF freedom," said lead investigator Stephan Willems, M.D., Hamburg, Germany. "Charge density mapping revealed conduction patterns outside of the pulmonary vein in all but two patients, and we were able to use the map, ablate, remap strategy to achieve exceptional outcomes. The fact that physicians were able to achieve such impressive results with no prior experience with charge density mapping is a huge testament to the extent that this technology can help physicians improve effectiveness. We're seeing patterns we've never seen before and the more we learn and understand these patterns, the better care we can provide."
AcQMap utilizes precision ultrasound atrial anatomy imaging and high-definition non-contact charge mapping to visually represent real-time electrical patterns in the left and right atria during complex arrhythmias. Each time a physician maps a patient's heart, in less than 60 seconds, a customized visual representation of the arrhythmia is captured and displayed. With improved insight and re-mapping capabilities, ablation strategy is informed and physicians can strive to improve outcomes by checking their work after each ablation.
AcQMap was cleared by the U.S. Food and Drug Administration (FDA) in 2017 and received CE Mark in 2016. The system now also provides contact mapping capabilities in Europe, which received CE Mark in April of this year.
For more information: www.acutusmedical.com
References
1. Mujović N., Marinković M., Lenarczyk R., et al. Catheter Ablation of Atrial Fibrillation: An Overview for Clinicians. Adv Ther. 2017;34(8):1897-1917. DOI: 10.1007/s12325-017-0590-z.


 April 24, 2025
April 24, 2025