April 9, 2025 — Merit Medical Systems has announced the U.S. commercial release of its Ventrax Delivery System.
Ventrax is intended to facilitate placement of devices used in ablation procedures commonly performed to treat an abnormally fast heartbeat known as ventricular tachycardia (VT). Ventricular arrhythmias, which include VT, are believed to cause approximately three out of four sudden cardiac deaths, which result in an estimated 184,000–450,000 lives lost in the United States each year.¹
The all-in-one device provides a streamlined pathway for ablation catheters to enter the left ventricle through the aorta, an approach known as retrograde aortic access.
“For the electrophysiologist, using retrograde aortic access was always a risk-benefit decision, and the community has needed a tool that would make that decision easier,” said Albert Sun, MD, electrophysiologist at Duke Health in Durham, NC, and whose advice was instrumental in Merit's development of the Ventrax system.
Retrograde aortic access can be useful to reach certain areas of the ventricle compared to traditional methods, such as transseptal puncture. Using this approach, these areas of the left ventricle are more accessible,² supporting targeted treatment.
“The new retrograde delivery system provides access to the left ventricle, allowing for the exchange of catheters to diagnose, map, or treat VT,” said Jason Koontz, MD, PhD, electrophysiologist at Duke Health and product development consultant to Merit. “This adds to our tools as we work to deliver the best possible outcomes for our patients.”
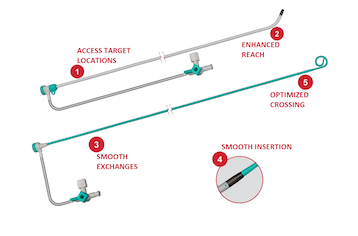
Key features of the Ventrax Delivery System include a 95-cm sheath designed to access desired target locations. An ultralow-profile transition between its sheath and pigtail-dilator offers smooth insertion, and an angled tip enhances the reach of an ablation catheter.
VT ablation is one of the fastest growing areas in electrophysiology. Many physicians are using retrograde aortic access for VT procedures, and researchers are increasingly investigating the technique.
The new delivery system is Merit’s latest addition to its electrophysiology (EP) and cardiac rhythm management (CRM) portfolio. The portfolio provides a unique selection of solutions to improve cardiac interventions, including the HeartSpan, Worley, Prelude SNAP and SafeGuard Focus product lines.
Learn more about the Ventrax Delivery System
- Khurshid et al. 2018. “Frequency of Cardiac Rhythm Abnormalities in a Half Million Adults.” Circ Arrhythm Electrophysiol 11 (7): e006273. (PMID: 29954742)
- Adlan et al. 2019. “Retrograde Aortic Access During Ventricular Tachycardia Ablation: Indications, Techniques, and Challenges.” Journal of Cardiovascular Electrophysiology 30 (11): 2629–2639. (PMID: 31502368)


 February 06, 2026
February 06, 2026 









