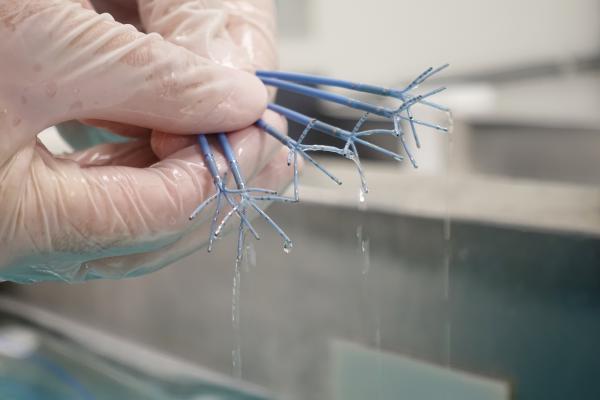
July 5, 2019 — Single-use cardiology medical device reprocessing company Innovative Health, received U.S. Food and Drug Administration (FDA) clearance for reprocessing the PentaRay Nav Eco High-Density mapping catheter manufactured by Biosense Webster. The PentaRay is a key medical device in atrial fibrillation (AF) procedures, one of the fastest growing and most expensive procedures in hospitals today, according to the company. Hospitals utilizing the reprocessed PentaRay and other expensive single-use labeled cardiology devices can save thousands of dollars on each AF procedure, potentially opening the procedure up for more patients.
The PentaRay catheter is an advanced technology whose capabilities include high-resolution mapping and coverage of all four chambers of the heart, which Biosense Webster associates with reduced procedure and fluoroscopy time.
In the past, the device geometry provided a challenge for reprocessing and use a second time, because it contains microlumen technology (channels the size of a human hair), and an FDA clearance requires cleaning and testing access to all areas of the device. In this instance, Innovative Health collaborated with the FDA through the pre-submission process to seek their guidance on appropriate test methods for reprocessing this device. Through this interaction, Innovative Health created entirely new protocols and standards for reprocessing the device. This included state-of-the-art processes for removing blood and heparin out of the microlumen, and rigorous testing to ensure that these efforts were effective. Innovative Health’s own protocols also involved ensuring that the device was compatible with the entire Carto 3 mapping system, also manufactured by Biosense Webster, and that it meets specifications equivalent to the new device.
The new standards and processes used to reprocess the PentaRay will allow Innovative Health to reprocess other similar devices using microlumen technology, which is becoming dominant in AF ablation procedures.
Innovative Health CEO Rick Ferreira said that the FDA clearance to reprocess PentaRay will make it possible for hospitals to reduce AF ablation medical device costs by as much as 30 percent.
For more information: www.innovative-health.com


 December 19, 2025
December 19, 2025 









