March 13, 2022 – A new way to replicate what happens inside the heart after cardiac arrest could open new avenues for ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
March 13, 2022 – People with a congenital heart defect who were hospitalized with COVID-19 infection were at higher risk ...
March 12, 2022 – In its 30th year of partnership with the American Heart Association, NYCM Insurance is proud to ...
March 12, 2022 – Charles D. Fraser, Jr., M.D., an internationally recognized congenital heart surgeon, has been named ...
March 11, 2022 – Cardiology Consultants of Philadelphia (CCP) — the region’s largest and most extensive cardiology ...
March 11, 2022 – Patients who survive a heart attack together with sudden cardiac arrest are at increased risk of dying ...
March 9, 2022 – According to the American Heart Association, scientific research supports the view that losing an hour ...

The University of Maryland Medical Center has announced that David Bennett, the 57-year old patient with terminal heart ...
March 9, 2022 — Fujifilm Sonosite, Inc., a world leader in point-of-care ultrasound (POCUS) solutions today announced it ...
March 8, 2022 – MemorialCare Heart & Vascular Institute (MHVI), through its advanced research program, is participating ...
Mrach 8, 2022 – Diagnosing heart attacks after heart surgery remains difficult due to shortcomings of current diagnostic ...
March 7, 2022 — AdvaMed is saddened to share that a beloved member of the AdvaMed family, Wendy Siminski, Senior Vice ...
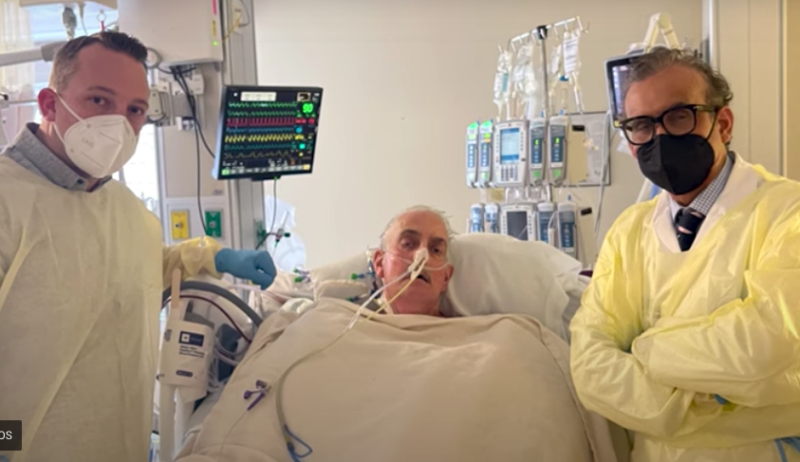
Here is what you and your colleagues found to be most interesting in the field of cardiology during the month of ...
March 4, 2022 – NewAmsterdam Pharma (NAP), a clinical-stage company focused on the research and development of ...
March 3, 2022 – Spontaneous coronary artery dissection (SCAD), a tear in an artery supplying blood to the heart, is a ...


 March 11, 2022
March 11, 2022