June 4, 2020 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) magaz ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.

The American College of Cardiology (ACC) together with other North American cardiovascular societies issued a framework ...

June 1, 2020 — The U.S. Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for the Abiom ...

May 29, 2020 — As states begin to re-open as the number of COVID-19 patients declines, 40 percent of Americans still ...
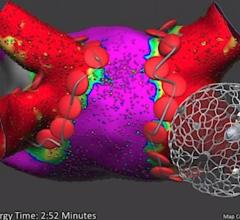
Jay Mohan, D.O., RPVI, interventional cardiology fellow at William Beaumont Hospital, Royal Oak, Michigan, created this ...
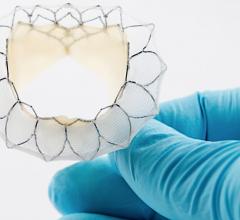
May 29, 2020 — Neovasc Inc., developer of a minimally invasive transcatheter mitral valve replacement (TMVR) ...
May 29, 2020 — Medtronic is recalling its HeartWare HVAD left ventricular assist device (LVAD) pump outflow graft and ...
May 28, 2020 — The rising incidence of cardiovascular disease has prompted people to seek timely treatment to improve ...
May 28, 2020 — As hospitals begin reopening to elective exams and procedures amid the COVID-19 pandemic, they need to be ...
May 27, 2020 — Carmat, a developer of the of a next generation advanced total artificial heart, announces the first ...
May 27, 2020 — To ensure the health and safety of all attendees due to the ongoing COVID-19 (SARS-CoV-2) pandemic, the C ...
Interview with Andrew D. Krahn, M.D., FHRS, head of the division of cardiology at St. Paul’s Hospital, and professor of ...
Andrew D. Krahn, M.D., FHRS, head of the division of cardiology at St. Paul’s Hospital, professor of medicine at the ...
May 21, 2020 — Two late-breaking clinical trials on trans catheter valves to treat congenital heart disease were ...

A new, serious COVID-19 cardiovascular presentation emerged in late April and early May 2020 in the form of pediatric ...

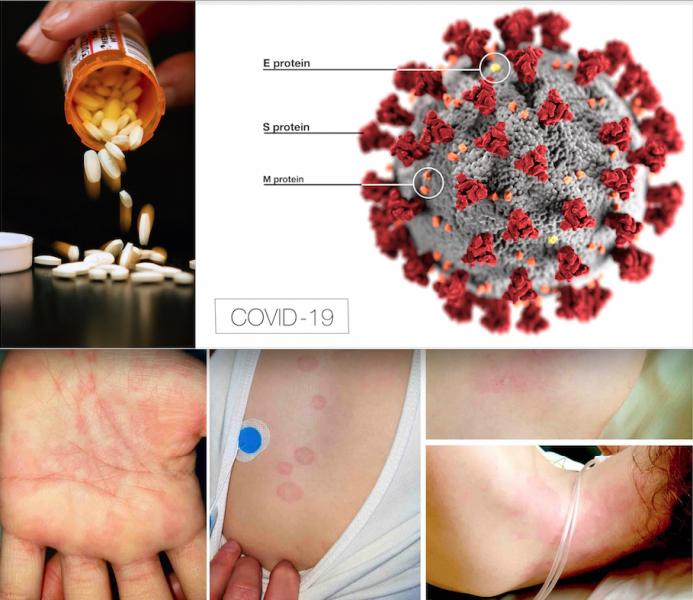
 June 04, 2020
June 04, 2020