May 15, 2021 — Patients with an elevated risk of stroke due to heart rhythm problems, or atrial fibrillation (AFib) ...
Diagnostic and Interventional Cardiology DAIC
Diagnostic and Interventional Cardiology (DAIC) is a magazine that reaches more than 25,000 healthcare professionals in cardiology, interventional cardiology and cath labs across the United States. These influential buying team members rely on DAIC's award-winning editorial content and comparison charts as a unique research tool for specifying, recommending and approving technology/device purchases.
May 15, 2021 — The anticoagulant apixaban (Eliquis) was not superior to standard of care following transcatheter aortic ...

There is a trend in interventional cardiology that is now being called “renalism,” where patients with poor renal ...
May 13, 2021 — The Centers for Disease Control and Prevention (CDC) just released a new statement relaxing the ...

The mitral valve anatomy is extremely complex, which has caused many challenges for transcatheter mitral valve ...
Tom Jones, M.D., director, cardiac catheterization laboratories, Seattle Children’s Hospital, and principle investigator ...
May 13, 2021 — Estimates of excess deaths, defined as the number of persons who have died from all causes, above the ...
May 13, 2021 — PolyVascular, leading the effort to improve children's lives with a novel polymeric transcatheter valve ...
May 12, 2021 — Preliminary results of a clinical trial, presented today at the AATS 101st Annual Meeting, showed that a ...

Heart failure remains an unheralded global pandemic, costing society billions of dollars each year.[1,2] Projections ...
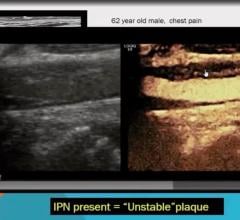
May 11, 2021 — The American Institute of Ultrasound in Medicine (AIUM) and the American Society of Echocardiography (ASE ...
May 11, 2021 — Patients hospitalized with COVID-19 may be at risk of developing heart failure even if they do not have a ...
Philippe Géneréux, M.D., director of the structural heart program at Atlantic Health System’s Morristown Medical Center ...
May 6, 2021 — The wearable cardiac devices market is set to grow from its current market was valued at more than $1.2 ...
Ashwin Nathan M.D., a cardiology fellow in the division of Cardiovascular Medicine at the Hospital of the University of ...

 May 15, 2021
May 15, 2021