July 12, 2018 — Intact Vascular Inc. announced the publication of the iDissection Classification trial results in the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
July 12, 2018 — CathWorks announced the approval of a new Current Procedural Terminology (CPT) code 0523T for non ...
Ori Ben-Yehuda, M.D., executive director of the Cardiovascular Research Foundation Clinical Trial Center, discusses the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
I attended the American Society of Echocardiography (ASE) 2018 meeting in June and have the following takeaways on ...
DAIC Editor Dave Fornell highlights some of the most innovative new technology on the show floor of the American Society ...

As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
Rebecca Hahn, M.D., professor of medicine and director of interventional echocardiography, Columbia University Medical ...
July 3, 2018 — HeartFlow Inc. announced that UnitedHealthcare now covers the HeartFlow FFRct Analysis, extending access ...
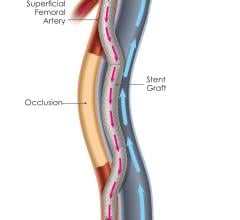
July 2, 2018 — The PQ Bypass Detour System showed promising 12-month durability for patients with extremely long ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
This is a demonstration of the the Philips TrueVue photo-realistic rendering and lighting source technology. This ...
June 25, 2018 — Computed tomography (CT) angiography is a useful adjunct to autopsy that is likely to increase the ...

About 50 percent of patients in cardiogenic shock do not survive, and account for about 90,000 heart attack patients a ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
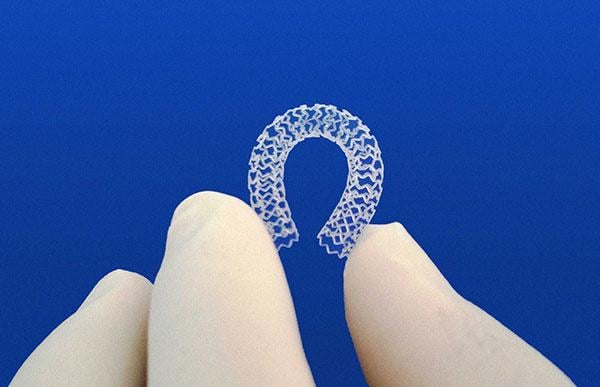
June 19, 2018 – Amaranth Medical offered new details about its 85-micron Defiance bioresorbable scaffold (BRS) recently ...
June 15, 2018 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for 200mm and 250mm lengths ...
June 14, 2018 — The U.S. Food and Drug Administration (FDA) released the following two draft guidance documents:
- Coro ...

 July 12, 2018
July 12, 2018