April 3, 2009 - Long-term data presented at ACC.09 from Abbott’s SPIRIT II clinical trial demonstrated the ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
The QuikClot Interventional dressing made by Z-Medica uses a mineral impregnated bandage to achieve hemostasis ...
April 2, 2009 - Volcano Corp. this week at ACC 2009 announced the availability of its integrated fractional flow ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
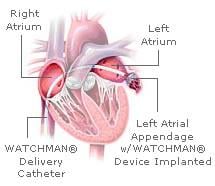
April 2, 2009 - A device implanted in the heart using minimally invasive techniques may replace the most widely ...
April 2, 2009 — Abbott yesterday announced the initiation of the MOBILITY clinical trial to study the safety and ...
April 2, 2009 – Invatec received 510(k) clearance from the FDA to market its Amphirion Deep 150 mm Long PTA ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
April 1, 2009 - Medtronic Inc. this week began the international launch of the Driver Sprint RX Coronary Stent ...
April 1, 2009 - Cardiac Data Solutions’ research findings presented at ACC this week report that the number of ...
March 31, 2009 - St. Jude Medical Inc. and GE Healthcare said at ACC that they have commercially launched the ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
March 30, 2009 – Improving upon the success of its current X-ray technologies, Toshiba America Medical Systems ...
March 30, 2009 - An AHRQ-funded study finds a lower risk of death and heart attack in heart disease patients 65 ...
March 30, 2009 – Analysis of Boston Scientific’s SYNTAX trial found that while the overall cost effectiveness of ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
March 30, 2009 - Twelve-month data from the high-risk registry arm of the EVEREST II study shows that ...
March 30, 2009 – Medtronic and the FDA announced a Class I recall of the Innervision Snap Shunt Ventricular ...
March 27, 2009 - The first data from PLC Systems’ pilot safety trial for RenalGuard will be presented in a poster ...


 April 03, 2009
April 03, 2009











