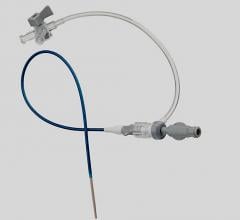
October 27, 2008 - Merit Medical Systems Inc., said last week it received 510(K) clearance from the FDA for the Prelude Short Sheath Introducer, which is used primarily for access to dialysis fistulas and grafts.
The company said the device provides several advantages for clinicians, including a marker tip to easily identify the exact placement of the device and an angled large-bore side arm for enhanced removal of blood clots. It will replace an existing product that is currently manufactured by an outside vendor.
Sales will commence worldwide in the next few weeks, the company said.
For more information: www.merit.com


 June 10, 2020
June 10, 2020