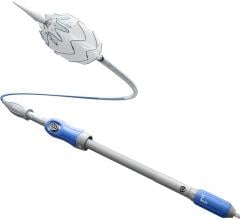
October 30, 2008 - C. R. Bard Inc. today received approval from the FDA to market the Flair Endovascular Stent Graft with an optimized delivery system.
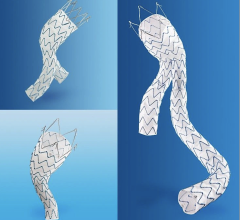
The device is comprised of a self-expanding Nitinol stent encapsulated within Bard’s proprietary ePTFE graft material. The Flair Endovascular Stent Graft is indicated to treat stenoses in synthetic arteriovenous bypass grafts. More than 300,000 patients with end-stage renal disease in the U.S. rely on these bypass grafts to receive hemodialysis treatment. These stenoses are the leading cause of bypass graft malfunction, compromising dialysis quality. Typically, multiple interventions, primarily with balloon angioplasty, are necessary to maintain the patency of bypass grafts over their useful lives.
Bard’s six month, prospective, randomized, pivotal study of 190 patients at 16 sites showed that placement of the FlairEndovascular Stent Graft resulted in more than twice the primary patency of balloon angioplasty (50.6 vs. 23.3 percent).
“The Flair Endovascular Stent Graft is the first interventional technology that has demonstrated superiority to balloon angioplasty for maintaining access patency,” said Ziv Haskal M.D., professor of radiology at the University of Maryland and lead investigator of the clinical trial.
This approval is Bard’s first in the U.S. peripheral vascular stent market. The company has two additional PMA applications pending, one for an iliac artery indication for its E-Luminexx Stent and another for a superficial femoral artery indication for the LifeStent FlexStar Stent.
For more information: www.crbard.com

 April 26, 2023
April 26, 2023