June 1, 2022 — Recardio's Phase 2 trial results demonstrated the excellent safety profile of its lead drug Dutogliptin ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
May 31, 2022 — Innovative Health, LLC, a specialty cardiology reprocessor, announced that the company has received ...
May 27, 2022 — Medtronic today announced approval from the U.S. Food and Drug Administration (FDA) for the IN.PACT 018 ...
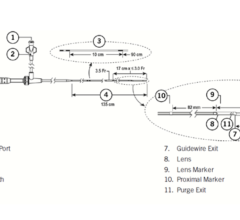
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
May 27, 2022 — The SELUTION SLR (Sustained Limus Release) is a novel sirolimus-eluting balloon that provides a ...
May 26, 2022 — The U.S. Food and Drug Administration (FDA) has recalled the Dragonfly OpStar Imaging Catheter, and has ...
May 25, 2022 — ŌNŌCOR LLC, a leader in endovascular safety technology, today announced it has received 510(k) U.S. Food ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
May 23, 2022 — New findings from the Ascension Health System’s internal National Cardiovascular Data Registry (NCDR) ...
May 20, 2022 — Results from a retrospective analysis reveal significantly higher mortality rates for low-income ...
May 20, 2022 — Results from a real-world study investigating safety and effectiveness of clopidogrel versus aspirin ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
May 20, 2022 — A new analysis of PROGRESS-CTO (PROspective Global REgiStry for the Study of Chronic Total Occlusion ...
May 20, 2022 — New long-term data from the Safety Assessment of Femoropopliteal Endovascular Treatment With PAclitaxel ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
May 19, 2022 — One year outcomes from the Disrupt PAD III Trial comparing intravascular lithotripsy (IVL) with a drug ...
May 19, 2022 — The latest analysis from The North American COVID-19 STEMI (NACMI) was presented today as late-breaking ...


 June 01, 2022
June 01, 2022