May 26, 2022 — The U.S. Food and Drug Administration (FDA) has recalled the Dragonfly OpStar Imaging Catheter, and has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
The Dragonfly OpStar imaging catheter with optical coherence tomography (OCT) imaging system is designed to provide imaging of blood vessels that carry blood and oxygen to the heart (coronary arteries) in people who are candidates for catheter-based, minimally invasive, interventional procedures to address coronary artery disease.
Reason for Recall
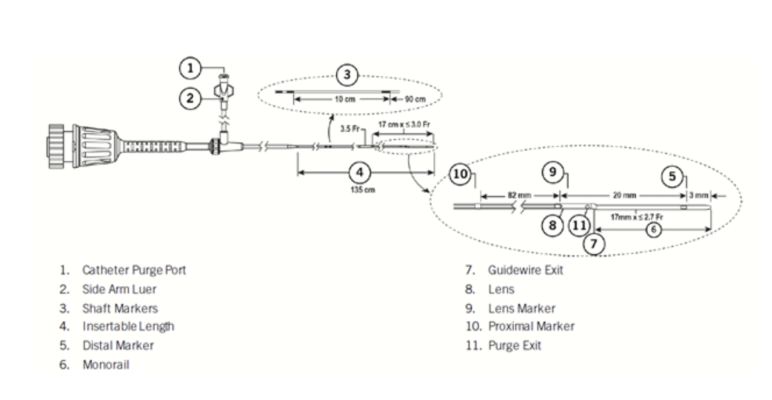
Abbott is recalling certain lots of the Dragonfly OpStar imaging catheter because the marker band farthest from the catheter tip (proximal marker) may become loose and, in two instances, has been observed to separate from the catheter while being used on a person.
A loose marker band that has separated from the device may remain in the body after the catheter is removed, potentially leading to vascular injuries, including but not limited to embolism (blockage of the vessel), thrombosis (blood clot), dissection (tear), ischemia (inadequate blood supply to the heart), infarction (heart attack), infection, or death.
There have been 5 incidents and 1 injury related to this device issue. No deaths have been associated with the use of this device due to this issue.
Who May be Affected
- Health care personnel who use the Dragonfly OpStar Imaging Catheter for OCT imaging of the coronary arteries.
- People who will have catheter-based OCT imaging of the coronary arteries using the Dragonfly OpStar Imaging Catheter. This issue does not impact people who have already had this procedure.
Recalled Product
-
Product Name: Dragonfly OpStar Imaging Catheter
-
Product Lot Numbers: See recall database entry
-
Devices Recalled in the U.S.: 4,800
-
Date Initiated by Firm: April 11, 2022
What to Do
On April 11, 2022, Abbott issued an Urgent Medical Device Recall letter to all customers who received affected devices. The following instructions were included:
- Immediately stop using devices from affected lots
- Review inventory and complete the Effectiveness Check Form Included with the Urgent Medical Device recall letter.
- Return all unused affected devices to Abbott.
- Share this information with all relevant personnel.
- Notify anyone who may have received these affected products through additional distribution or transfer.
- Report any product performance issues or adverse events to Abbott.
Contact Information
Customers with questions or concerns about this recall should contact their local Abbott representative or call the customer service department at 800-227-9902.
Related Dragonfly OpStar Imaging Catheter information:
FDA Clears Abbott OCT Imaging Enhanced With Artificial Intelligence
Abbott’s New Artificial Intelligence OCT Coronary Imaging Platform Launches in Europe


 November 14, 2025
November 14, 2025 









