June 15, 2012 — Stentys SA, a medical technology company commercializing the world's first and only self-apposing stent ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
June 15, 2012 — Cardiosolutions Inc. announced it has received 510(k) clearance from the U.S. Food and Drug ...

June 14, 2012 — A U.S. Food and Drug Administration (FDA) advisory panel voted in favor of recommending expanding the ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
June 14, 2012 — Abiomed Inc. said today it received Health Canada approval to market the Impella cVAD (ventricular ...
June 13, 2012 — Physicians presented at EuroPCR 2012 the results of two multicenter, randomized controlled trials, the ...
June 8, 2012 — Austin Heart, the largest provider of cardiac and vascular services in central Texas, announced its ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
June 7, 2012 — GE Healthcare announced the acquisition of XPRO, a Brazilian Interventional X-ray equipment manufacturer ...
June 5, 2012 — Hansen Medical Inc. received 510(k) market clearance from the U.S. Food and Drug Administration (FDA) for ...

Evaluating the performance of your cath lab is key to developing a strategic plan for improving service offerings ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
June 4, 2012 — SentreHeart Inc., a privately held medical device company, announced that it has recently completed a $26 ...
June 4, 2012 — A jury awarded $6.4 million to the young children of a Philadelphia man after finding that emergency room ...
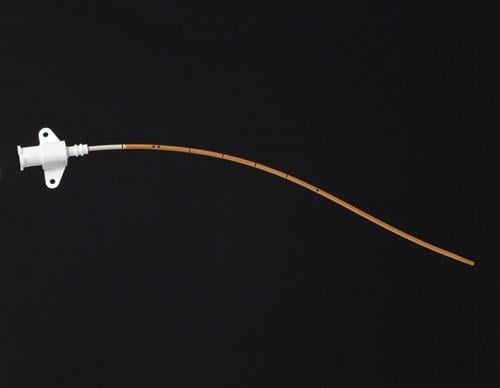
Peripherally inserted central catheters (PICCs) are devices used for intravenous access to facilitate the delivery of ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...
June 4, 2012 — Boston Scientific Corp. has received U.S. Food and Drug Administration (FDA) regulatory approval of 32 mm ...
Siemens' syngo Aortic ValveGuide software enables transcatheter aortic valve replacement (TAVR) procedural guidance with ...
June 1, 2012 — Kona Medical Inc. announced it has raised $30 million in Series C financing to advance its novel ...

 June 15, 2012
June 15, 2012








