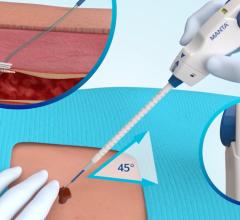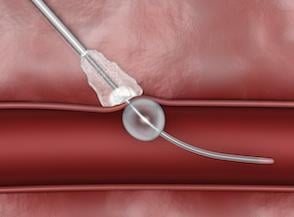
February 28, 2012 — AccessClosure Inc. announced the U.S. Food and Drug Administration (FDA) approval and U.S. launch of the MynxGrip Vascular Closure Device (VCD). Built on the Mynx platform, the MynxGrip VCD offers an active, extravascular and patient-friendly vascular closure solution. MynxGrip adds the proprietary Grip Technology sealant to the distal end of the original Mynx Sealant, resulting in a sealant that actively grips and seals the arteriotomy while expanding and filling the tissue tract, providing a durable hemostasis.
"There has been a long-standing perception that closure devices which 'actively' close the arteriotomy with sutures or clips provide greater security and have better results. Unfortunately the tradeoff for these closure devices has been leaving foreign material permanently behind in the artery," said Stevan Himmelstein, M.D., a cardiologist at Baptist Memorial Hospital-DeSoto in Southaven, Miss. "MynxGrip is the first closure device which provides the benefits of an active, secure close while also being completely extravascular and bioabsorbable."
The MynxGrip Sealant consists of the same polyethylene glycol (PEG) polymer used in the original Mynx Sealant. Grip Technology is a new configuration of PEG that adheres to the contours of the vessel wall, providing active closure of the arteriotomy. The MynxGrip Sealant fully resorbs within 30 days.
"We are extremely excited about the launch of MynxGrip," said Gregory D. Casciaro, president and CEO of AccessClosure Inc. "The total potential worldwide market for vascular closure devices includes an estimated nine million procedures each year. We believe the MynxGrip's enhanced extravascular sealant will be widely adopted by a customer base that seeks an active closure method but does not want to leave anything behind in the vessel."
For more information: www.accessclosure.com


 October 07, 2025
October 07, 2025