PARADISE
February 20, 2012 — ReCor Medical announced that its Paradise percutaneous ultrasound renal denervation system for achieving rhas received the CE mark. Paradise is designed to treat patients with resistant hypertension (HTN), a major risk factor for cardiovascular disease.
The F-I-M clinical data for Paradise was reported at the TRenD 2012 transcatheter renal denervation scientific meeting by cardiologist Thomas A. Mabin, M.D., Vergelegen Medi-Clinic, South Africa. The Paradise data showed that systolic blood pressure was reduced by an average of 31 mm Hg in seven patients at 60-day follow-up.
“Paradise designed to offer a minimally invasive ultrasound therapy to resistant hypertension patients to help reduce their blood pressure,” said Mano Iyer, CEO, ReCor Medical. “We are extremely pleased with our first-in-man clinical results as we prepare to launch Paradise in Europe.”
“The initial results with Paradise are impressive,” added professor Marc Sapoval, Hôpital Européen Georges-Pompidou, Paris, who is a member of ReCor Medical’s Medical Advisory Board. “This degree of blood pressure reduction has significant health benefits for patients.”
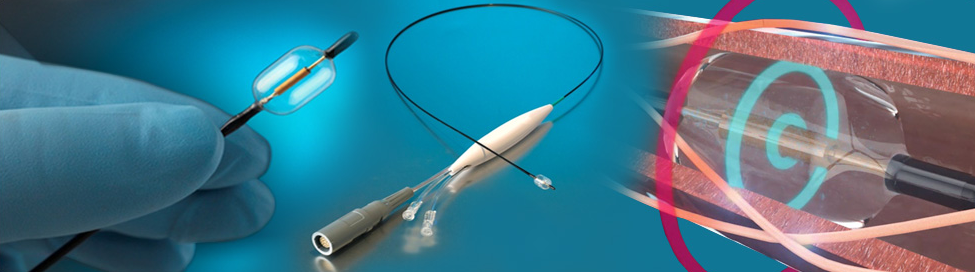
Paradise includes a 6 French-compatible catheter with a cylindrical transducer that emits ultrasound energy circumferentially, allowing for a rapid and highly efficient renal denervation procedure. The advantage of Paradise is its ability to uniformly denervate all the way around the arterial wall while simultaneously cooling the endothelium, to help enable a safe, consistent, and fast renal denervation procedure.
Paradise is approved for sale in Europe; it is not approved for sale or investigational use in the United States.
For more information: www.recormedical.com


 February 06, 2026
February 06, 2026 









