December 20, 2012 — New streamlined guidelines will help healthcare providers better treat patients with the most severe ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
December 19, 2012 — Merit Medical Systems Inc. announced that it has received 510(k) clearance from the U.S. Food and ...
December 19, 2012 — CardiacAssist Inc. announced it has received investigational device exemption (IDE) approval from ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
DAIC Editor Dave Fornell highlights the latest advancements that will impact cardiovascular imaging from the 2012 ...
December 17, 2012 — Abiomed Inc. announced the U.S. Food and Drug Administration's (FDA) Circulatory System Devices ...
December 13, 2012 — St. Jude Medical Inc. announced the first patient implant of its 23 mm Portico transcatheter aortic ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
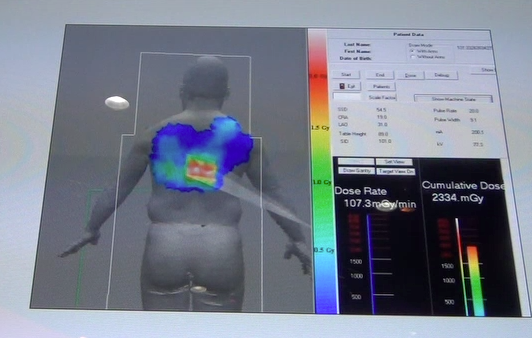
The latest advances in cardiovascular imaging are usually shown first at the Radiological Society of North America (RSNA ...
December 10, 2012 — At RSNA 2012, GE Healthcare showcased a number of new advanced imaging solutions for the surgical ...
December 10, 2012 — Vascular Closure Systems Inc. announced the successful conclusion of Phase I and Phase II of the ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
December 10, 2012 — The U.S. Food and Drug Administration (FDA) is notifying healthcare professionals, caregivers and ...
December 7, 2012 — More than half of patients treated for claudication or critical limb ischemia (CLI) with stenting of ...
December 7, 2012 — Featuring hovercraft-like C-arm movement and the unique Access Halo, the WorkRite technology on ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

December 7, 2012 — Tryton Medical Inc. announced the completion of enrollment in the TRYTON Pivotal U.S. Food and Drug ...
One-year results from the ADAPT-DES Trial were presented during TCT 2012. It examined patient hyporesponsiveness to ...
December 7, 2012 — Volcano Corporation announced that it has entered into a definitive agreement to acquire Sync-Rx Ltd ...

 December 20, 2012
December 20, 2012